Research Projects
REGULATION OF THE NOTCH SIGNALING PATHWAY BY O-GLYCOSYLATION
1. BACKGROUND OF THE RESEARCH
1-1. Discovery of O-glycosylation as an essential regulator for Notch signaling
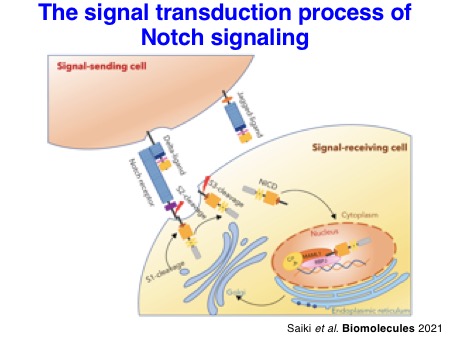
After the pioneering work on the discovery of O-fucose and O-glucose glycans reported by the Haltiwanger group, the accumulating evidence indicates that O-linked glycosylation is an essential regulator of Notch signaling. Notch signaling is an evolutionarily well-conserved signaling pathway for cell fate decision in metazoan. Not surprisingly, dysregulation of this intercellular signaling pathway leads to many human diseases.
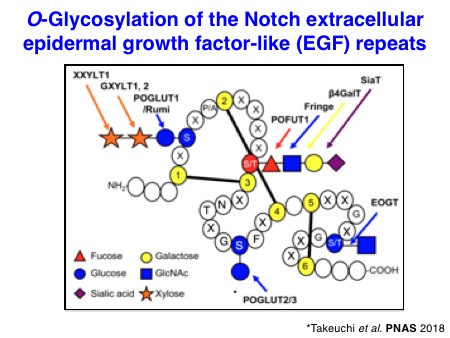
There are 29-36 epidermal growth factor-like repeats in the extracellular domain of Notch receptors. EGF repeats can be modified with three different types of O-linked glycans such as O-fucose, O-glucose, and O-GlcNAc, at a specific position of EGF repeats. These monosaccharides can be further extended by specific glycans. Of note, the biosynthesis of glycans is generally catalyzed by a specific sugar-building enzyme, glycosyltransferase.
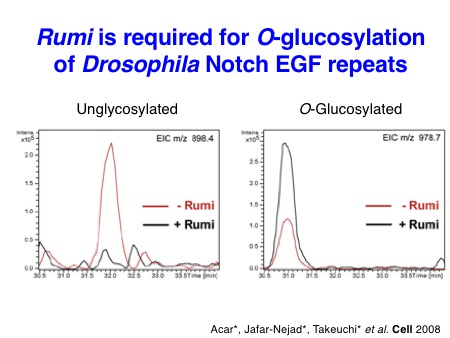
Particularly, we contributed to the demonstration of the importance of O-glucose glycans for Notch signaling in the early studies. In collaboration with the Bellen lab and the Jafar-Nejad lab, we identified a long-sought protein O-glucosyltransferase POGLUT1 (rumi in Drosophila) as an essential component for Notch signaling in Drosophila and mice. Subsequently, we also discovered two other protein O-glucosyltransferase, POGLUT2 and POGLUT3, with a distinct acceptor specificity.
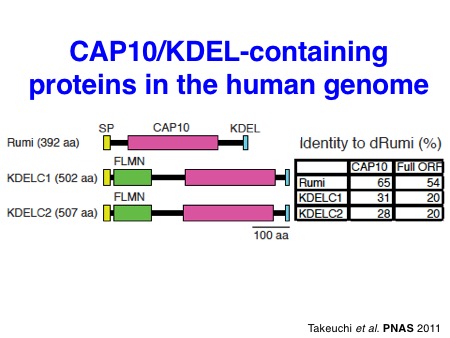
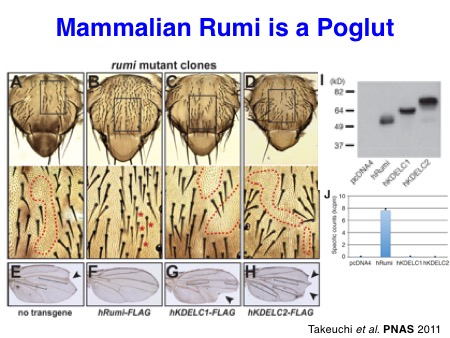
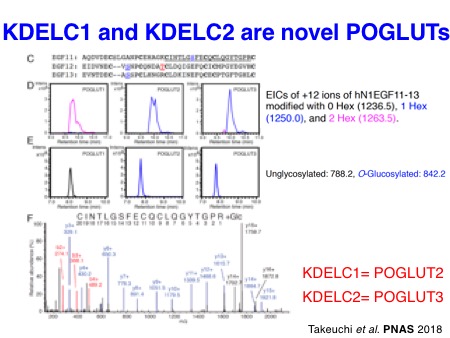
Takeuchi H*, Schneider M*, Williamson DB, Ito A, Takeuchi M, Handford PA, and Haltiwanger RS. Two novel protein O-glucosyltransferases that modify sites distinct from POGLUT1 and affect Notch trafficking and signaling.
Proc Natl Acad Sci USA 2018 Sep 4; 115 (36): E8395-402.
Takeuchi H, Fernandez-Valdivia RC, Caswell DS, Nita-Lazar A, Rana NA, Macnaughtan MA, Jafar-Nejad H, and Haltiwanger RS. Rumi functions as both a protein O-glucosyltransferase and a protein O-xylosyltransferase.
Proc Natl Acad Sci USA 2011 Oct; 108 (40): 16600-5.
Fernandez-Valdivia RC, Takeuchi H, Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS, and Jafar-Nejad H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi.
Development 2011 May; 138 (10): 1925-34.
Acar M*, Jafar-Nejad H*, Takeuchi H*, Rajan A, Ibrani D, Rana NA, Pan H, Haltiwanger RS, and Bellen H. Rumi, a CAP10 domain protein, is a glycosyltransferase that modifies Notch and is required for Notch signaling.
Cell 2008 Jan; 132 (2): 247-58. (Previewed in Cell 2008 132(2): 177-9, Highlighted in Functional Glycomics (14 Feb 2008), Recommended by Faculty of 1000)
1-2. Human diseases linked to aberrant O-glycosylation in Notch receptors
There are several mutations found in the genes of Notch-modifying glycosyltransferases as summarized in the table. Through the international collaboration directed by Dr. Carmen Paradas, we recently reported that autosomal recessive mutations in POGLUT1 cause limb-girdle muscular dystrophy (LGMD) R21 and that POGLUT1-dependent Notch signaling is important for the maintenance of satellite cells, muscle stem cells, and, to our surprise, the glycosylation of alpha-dystroglycan produced in differentiated myofibers. The number of disease types and genetic mutations in the Notch-modifying glycosyltransferase genes to be listed is still growing.
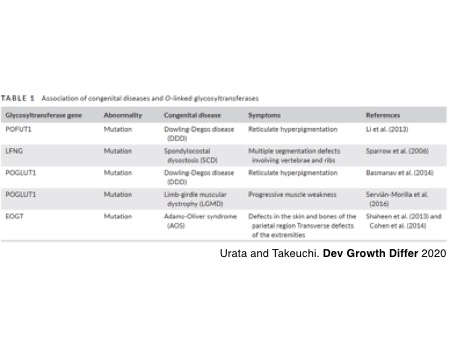
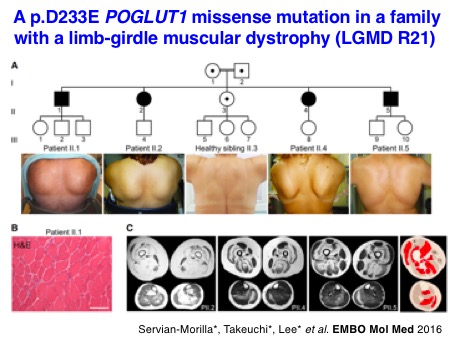
Servian-Morilla E, Cabrera-Serrano M, Johnson K, Pandey A, Ito A, Rivas E, Chamova T, Muelas N, Mongini T, Nafissi S, Claeys KG, Grewal RP, Takeuchi M, Hao H, Bonnemann C, Lopes Abath Neto O, Medne L, Brandsema J, Topf A, Taneva A, Vilchez JJ, Tournev I, Haltiwanger RS, Takeuchi H, Jafar-Nejad H, Straub V, and Paradas C. POGLUT1 biallelic mutations cause myopathy with reduced satellite cells, α-dystroglycan hypoglycosylation and a distinctive radiological pattern.
Acta Neuropathol 2020 Mar; 139 (3): 565-82.
Ralser DJ, Takeuchi H, Fritz G, Basmanav FB, Effern M, Sivalingam S, El-Shabrawi-Caelen L, Degirmentepe EN, Kocaturk E, Singh M, Booken N, Spierings NMK, Schnabel V, Heineke A, Knuever J, Wolf S, Wehner M, Tronnier M, Leverkus M, Tantcheva-Poor I, Wenzel J, Oji V, Has C, Holzel M, Frank J, Haltiwanger RS, and Betz RC. Altered Notch signaling in Dowling-Degos disease: Additional mutations in POGLUT1 and further insights into disease pathogenesis.
J Invest Dermatol 2019 139: 960-964.
Takeuchi H, Wong D, Schneider M, Freeze HH, Takeuchi M, Berardinelli SJ, Ito A, Lee H, Nelson SF, and Haltiwanger RS. Variant in human POFUT1 reduces enzymatic activity and likely causes a recessive microcephaly, global developmental delay with cardiac and vascular features.
Glycobiology 2018 May; 28 (5): 276-83. (Recommended by Faculty of 1000 Prime)
Servian-Morilla E*, Takeuchi H*, Lee TV*, Clarimon J, Mavillard F, Area-Gomez E, Rivas E, Nieto-Gonzalez JL, Rivero MC, Cabrera-Serrano M, Gomez-Sanchez L, Martinez-Lopez JA, Estrada B, Marquez C, Morgado Y, Suarez-Calvet X, Pita G, Bigot A, Gallardo E, Fernandez-Chacon R, Hirano M, Haltiwanger RS, Jafar-Nejad H, Paradas C. A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss.
EMBO Mol Med 2016 Nov; 8 (11): 1289-1309.
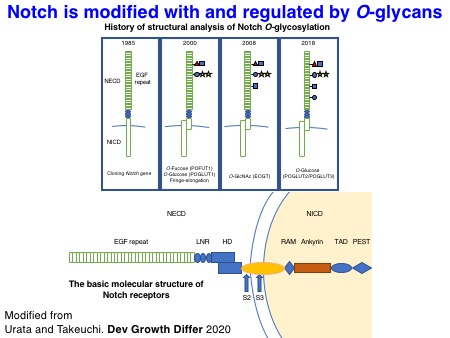
1-3. Structural basis of Notch O-glycosylation
The biochemical properties of Notch-modifying glycosyltransferases are unique and interesting. In collaboration with the Li lab, we succeeded in solving the X-ray structures of Drosophila rumi/POGLUT1 complexed with acceptor EGF repeats for the first time in 2016. Subsequently, the Rini lab in the University of Toronto revealed X-ray structures of human POGLUT1 in complex with acceptor EGF repeats in 2017. Both Drosophila and human POGLUT1 proteins have similar structures. Importantly, the structural information of POGLUT1 provides fundamental insights into the recognition mode of properly folded EGF repeats by POGLUT1.
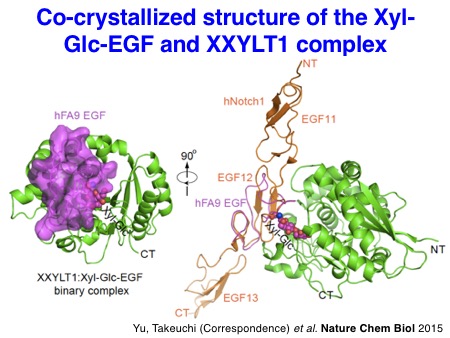
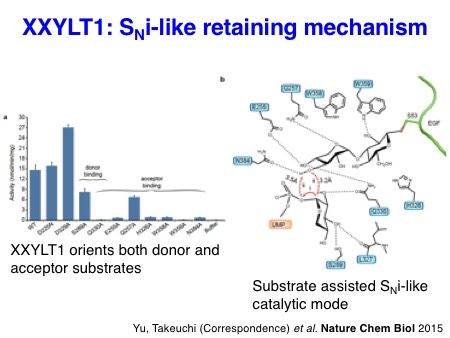
We also succeeded in solving the X-ray structures of mouse XXYLT1 in complex with acceptor EGF repeats modified with a Xyl-Glc disaccharide for the first time in 2015. This work provided the structure of a biologically important enzyme that is a potential drug target, solved a long-standing debate about the mechanism of retaining glycosyltransferases, and provided novel insights into the regulation of Notch signaling by targeted glycosylation.
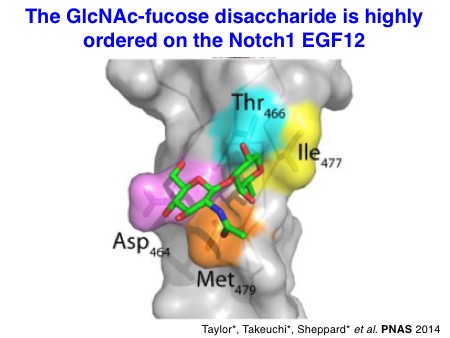
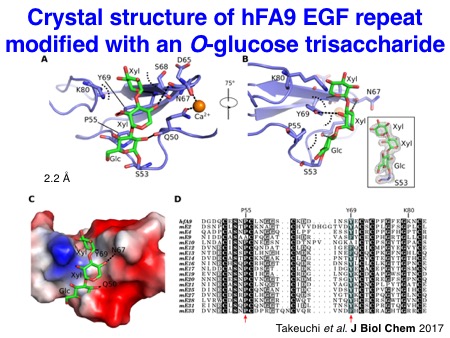
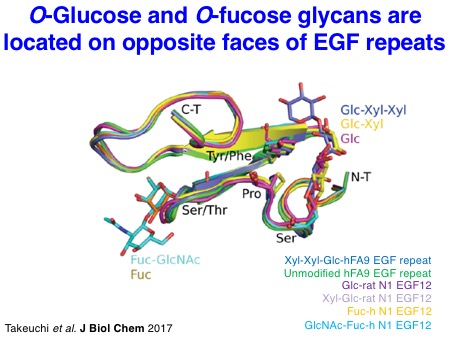
We have also investigated the structures of glycosylated EGF repeats. In collaboration with the Handford lab and the Lea lab at the University of Oxford, we could visualize the X-ray structures of NOTCH1 EGF11-13 proteins modified with an O-fucose disaccharide. Similarly, we solved the X-ray structures of a single EGF repeat modified with an O-glucose trisaccharide with the significant effort made by Dr. Hongjun Yu. It turned out that O-glucose and O-fucose glycans are located on the opposite faces of EGF repeats. Notably, these glycans are highly ordered on the EGF repeats through the intramolecular interactions, which should stabilize the overall structures of Notch EGF repeats.
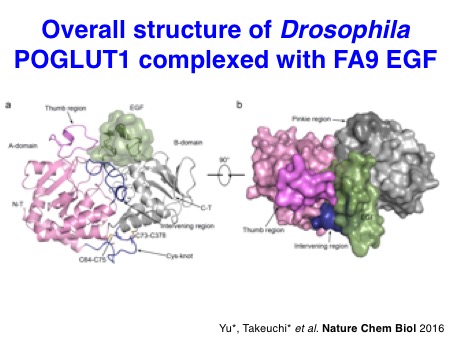
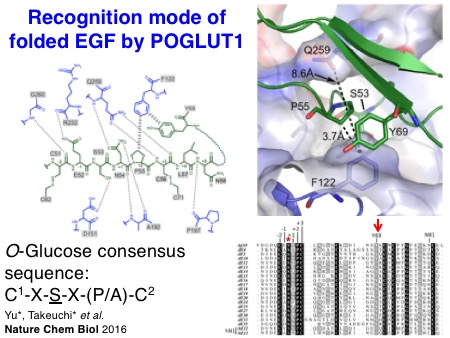
Yu H*, Takeuchi H*, Takeuchi M, Kantharia J, Haltiwanger RS#, and Li H#. Structural analysis of Notch-regulating Rumi reveals basis for pathogenic mutations.
Nature Chem Biol 2016 Sep; 12 (9): 735-40.
Valero-Gonzalez J*, Leonhard-Melief C*, Lira-Navarrete E, Jimenez-Oses G, Hernandez-Ruiz C, Pallares CP, Yruela I, Vasudevan D, Lostao A, Corzana F, Takeuchi H, Haltiwanger RS, and Hurtado-Gerrero R. A proactive role of water molecules in acceptor recognition by Protein-O-fucosyltransferase 2.
Nature Chem Biol 2016 Apr; 12 (4): 240-6.
Yu H, Takeuchi M, Kantharia J, LeBarron J, London E, Bakker H, Haltiwanger RS, Li H#, and Takeuchi H#. Notch-modifying xylosyltransferase structures support an SNi-like retaining mechanism.
Nature Chem Biol 2015 Nov; 11 (11): 847-54.
Taylor P*, Takeuchi H*, Sheppard D*, Chandramouli C, Lea SM, Haltiwanger RS, and Handford PA. Fringe-mediated extension of O-linked fucose in the ligand-binding region of Notch1 increases binding to mammalian Notch ligands.
Proc Natl Acad Sci USA 2014 May; 111 (20): 7290-5.
Sethi, MK, Buettner FFR, Ashikov A, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R, and Bakker H. Moleculer cloning of a xylosyltransferase that transfers the second xylose to O-glucosylated epidermal growth factor repeats of Notch.
J Biol Chem 2012 Jan; 287 (4): 2739-48.
Sethi MK, Buettner FF, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R, and Bakker H. Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats.
J Biol Chem 2010 Jan; 285 (3): 1582-6.
1-4. Molecular mechanisms by which O-glycosylation regulates Notch activation
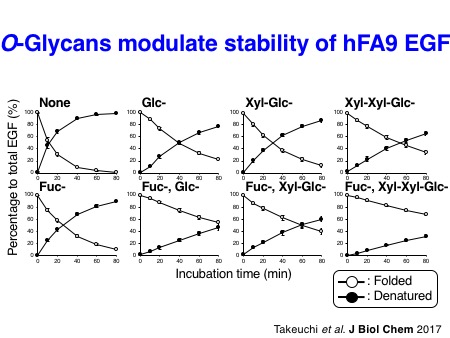
How do these O-glycans regulate Notch activation? N-Linked glycosylation is known to promote the folding of proteins. Interestingly, the glycosyltransferases, POFUT1, POGLUT1, and EOGT that catalyze the addition of O-fucose, O-glucose, and O-GlcNAc, respectively, utilize only properly folded EGF repeats as acceptor substrates. This notion suggests that these enzymes sense the status of protein folding of Notch EGF repeats. Structural analyses of EGF repeats modified with O-glycans suggested that EGF repeats can be stabilized by O-glycans. Further biochemical analyses supported this notion. Thus, these experimental findings strongly suggest that O-glycans play a role in protein quality control of Notch receptors.
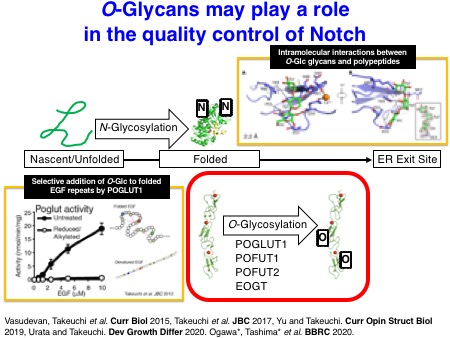
Urata Y, Saiki W, Tsukamoto Y, Sago H, Hibi H, Okajima T, and Takeuchi H. Xylosyl extension of O-glucose glycans on the extracellular domain of NOTCH1 and NOTCH2 regulates Notch cell surface trafficking.
Cells 2020 May 14; 9 (5): E1220.
Schneider M, Kumar V, Nordstrom LU, Feng L, Takeuchi H, Hao H, Luca VC, Garcia KC, Stanley P, Wu P, and Haltiwanger RS. Inhibition of Delta-induced Notch signaling using fucose analogs.
Nature Chem Biol 2018 Jan; 14 (1): 65-71.
Takeuchi H*, Yu H*, Hao H, Takeuchi M, Ito A, Li H, and Haltiwanger RS. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking.
J Biol Chem 2017 Sep; 292 (38): 15964-73.
Vasudevan D, Takeuchi H, Johar SS, Majerus E, and Haltiwanger RS. Peters plus syndrome mutations disrupt a non-canonical ER quality control mechanism.
Curr Biol 2015 Feb; 25 (3): 286-95.
Takeuchi H, Kantharia J, Sethi, MK, Bakker H, and Haltiwanger RS. Site-specific O-glucosylation of the epidermal growth factor-like (EGF) repeats of Notch: efficiency of glycosylation is affected by proper folding and amino acid sequence of individual EGF repeats.
J Biol Chem 2012 Oct; 287 (41): 33934-44.
Rana NA*, Nita-Lazar A*, Takeuchi H, Kakuda S, Luther KB, and Haltiwanger RS. O-Glucose trisaccharide is present at high but variable stoichiometry at multiple sites on mouse Notch1.
J Biol Chem 2011 Sep; 286 (36): 31623-37. (*: Equal contribution)
Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, Haltiwanger RS, and Irvine KD. In vitro reconstitution of the modulation of Drosophila notch-ligand binding by fringe.
J Biol Chem 2007 Nov; 282 (48): 35153-62.
REVIEWS and BOOK CHAPTERS
Saiki W, Ma C, Okajima T, and Takeuchi H. Current views on the roles of O-glycosylation in controlling Notch-ligand interactions.
Biomolecules 2021 Jan; 11 (2): 309.
Tsukamoto Y and Takeuchi H. Other types of glycosylation.
The roles of glycosylation in health and diseases 2021 IN PRESS.
Urata Y and Takeuchi H. Effects of Notch glycosylation on health and diseases.
Dev Growth Differ 2020 Jan; 62 (1): 35-48.
Yu H and Takeuchi H. Protein O-glucosylation: another essential role of glucose in biology.
Curr Opin Struct Biol 2019 Jan; 56: 64-71.
Takeuchi H and Haltiwanger RS. Significance of glycosylation in Notch signaling.
Biochem Biophys Res Commun 2014 Oct; 453 (2): 235-42.
Takeuchi H and Haltiwanger RS. Role of glycosylation of Notch in development.
Semin Cell Dev Biol 2010; Aug; 21(6): 638-45.
Note: A full list of Dr. Takeuchi’s publications can be found in his CV.
2. SPECIFIC AIMS IN THE CURRENT PROJECTS
Based on the results of the above studies on Notch signaling and glycosylation by us and other groups, we are planning the following studies.
Molecular mechanisms of regulation of Notch activation by O-glucose glycans in mammals
Rationale: Mutations in POGLUT1 cause human diseases such as muscular dystrophy and Dowling-Degos disease with dysregulation of Notch signaling, indicating the importance of O-glucosylation in human health.
Goals: We will analyze the overall structures of Notch and the other targets with O-glucose glycans in vivo by state-of-the-art technique, mass spectrometry. Furthermore, we will investigate how O-glucose glycans mechanistically regulate the activation of Notch receptors in different contexts.
Significance: A better understanding of molecular mechanisms of regulation of Notch activation by O-glucose glycans will contribute to basic science as well as clinical medicine.
Roles of O-glucose glycans in Notch-related cancers
Rationale: Dysregulation of Notch signaling is involved in a lot of different types of cancer. Notch signaling acts oncogenically in many cancers, but tumor-suppressively in some others. O-Glucosylation is essential for Notch signaling in model organisms (e.g. Drosophila and mice). Thus, O-glucosylation must also be involved in cancer.
Goals: We will analyze the role of O-glucosylation for Notch-related cancers in which O-glucosylation is dysregulated.
Significance: The data may suggest new preventive, diagnostic, and therapeutic strategies for the clinical and pharmacological treatment of cancer. Moreover, fundamental insights into cell autonomy and context-dependence of Notch signaling in vivo will be presented.
Structure/function analysis of Notch glycosyltransferases
Rationale: Fringe-dependent elongation of O-fucose with GlcNAc occurs at the specific EGF repeats and regulates Notch-ligand interactions. Genetic deletion of xylosyltransferases at least in Drosophila indicates that xylosyl-elongation inhibits Notch signaling in some contexts. The structural information of xylosyl-elongation of O-glucose in Notch receptors is limited.
Goals: We will analyze how xylosyl-elongation of O-glucose attached to the EGF repeats in Notch receptors occurs. Mammals have two glucoside xylosyltransferases, GXYLT1 and GXYLT2, while Drosophila has a sole enzyme Shams. There is a sole xyloside xylosyltransferase XXYLT1 in both mammals and Drosophila. The biochemical specificities and 3D structures of these enzymes will be analyzed.
Significance: The results of this project will clarify the regulatory mechanism in the biosynthesis of site-specific xylosyl-elongation and its effect on Notch signaling. Furthermore, the data will provide important insights into the reaction mechanism of retaining glycosyltransferases.
Development of inhibitors of Notch glycosyltransferases
Rationale: Notch signaling is involved in many biological processes. Different kinds of inhibitors of Notch signaling have been developed by targeting the Notch-ligand interactions, the processing of Notch receptors, and the function of the intracellular domain of Notch receptors. The biosynthesis of O-glycans in Notch receptors is catalyzed by the glycosyltransferases in the endoplasmic reticulum and Golgi apparatus through the secretory pathway during the transit of Notch.
Goals: We will explore inhibitors of Notch-modifying glycosyltransferases by taking advantage of the structural data of the glycosyltransferases and our expertise on the biochemical analysis of the glycosyltransferases.
Significance: The data will provide unique tools for regulating Notch signaling, which should contribute to the broad range of science.
3. ASPIRATIONS OF RESEARCH AND EDUCATION THROUGH FRUITFUL INTERNATIONAL COLLABORATION
Dr. Takeuchi would like to sincerely and actively engage in the pursuit of the truth of life and drug discovery-oriented pharmaceutical education. He had been engaged in research and education at the Graduate School of Pharmaceutical Sciences, the University of Tokyo (Dr. Tatsuro Irimura) from 1995-2006, Stony Brook University from 2006-2015 and the University of Georgia (Dr. Robert S. Haltiwanger) in the United States from 2015-2017, Nagoya University (Dr. Tetsuya Okajima) from 2017-2021. He will be most pleased to see undergraduate students and graduate students grow and become independent. It is our policy to do our best to support the career development of individual students in the Department of Biochemistry. Dr. Takeuchi believes that not only conducting research but also presenting the results to the world and contributing to society is an important part of research activities, so he provides students with many opportunities to present their research at academic conferences and focus on cultivating the ability to have their own opinions and communicate them to others.
It is very important to look at things from multiple aspects, and Dr. Takeuchi has felt firsthand that this is also true in the field of science. Therefore, he would like to actively continue to interact with professors from different fields inside and outside of the university and aim for a higher level in science with hard work and enthusiasm. Fortunately, he has had a chance to work with excellent international collaborators, some of whom are Dr. Hamed Jafar-Nejad (Baylor College of Medicine), Dr. Huilin Li (Van Andel Research Institute), Dr. Carmen Paradas (Instituto de Biomedicina de Sevilla), Dr. Hongjun Yu (Tongji Medical College, Huazhong University of Science and Technology), and Dr. Penny A. Handford (The University of Oxford). This international framework has been cultivated mainly during his 11-year stay in the United States. He has benefited greatly from this network, not only in the pursuit of truth in research but also in the pursuit of enrichment in life. He would like to share this international environment with younger people than himself to further strengthen and develop it.
Lab Events
April

April 2015 Cherry-blossom viewing party
Welcome party for new members
May - July
Sports events (Badminton, Softball)
Barbeque
Conference presentation (The Japanese Biochemical Society Chubu Branch、 The Pharmaceutical Society of Japan Tokai Branch)
August - September
Lab trip

Lab trip on August 18 2015(Higashi-izu、Odawara)
August - December
Conferecne presentation
(Annual Meeting of the Japanese Biochemical Society, the japanese society of Carbohydrate Research, the Japanese Society for Virology, the Pharmaceutical Society of Japan Tokai Branch, etc)
November
Open Lab in campus festival
Meeting of laboratory alumni
December
Lab's year-end party

Lab's year-end party on December 27th 2013
Graduation Thesis Presentation of 6-year Bachelor of Science in Pharmaceutical Sciences program
January
Doctoral Thesis Presentation
February
Master's Thesis Presentation
Graduation Thesis Presentation of 6-year Bachelor of Science in Pharmaceutical Sciences program
March
Graduation ceremony
Conferecne presentation(Annual Meeting of the Pharmaceutical Society of Japan)
Weekly meeting
Journal club
Lab meeting
Study group
History of the Department of Biochemistry
The Department of Biochemistry was established in 1953 when Shizuoka College of Pharmacy was founded. The Department of Biochemistry has developed through research on mouse breast cancer by Professor Junzo Kosaka (appointed in 1953), lipid biochemistry by Professor Makoto Matsumoto (appointed in 1962), and glycovirology by Professor Yasuo Suzuki (appointed in 1989) and Professor Takashi Suzuki (appointed in 2006). During this period, Shizuoka College of Pharmacy became University of Shizuoka School of Pharmaceutical Sciences which opened in 1987. Based on this world-renowned tradition of continuous research, four members of our department, Professor Hideyuki Takeuchi (appointed in 2021), Associate Professor Tadanobu Takahashi, Lecturer Akira Minami, and Assistant Professor Yuki Kurebayashi, are currently conducting cutting-edge glycobiology research with the aim of elucidating the fundamental nature of glycan functions and their application to innovative and original drug discovery.
1953
The Department of Biochemistry was established when Shizuoka College of Pharmacy opened.
April 1954

Junzo Kosaka became the first professor.
November 1962

Makoto Matsumoto became the second professor
October 1989

Yasuo Suzuki became the third professor (Currently Visiting Professor at Chubu University)
April 2006

Takashi Suzuki became the fourth professor
April 2021

Hideyuki Takeuchi became the fifth professor
