Research Achievements
Publications TOC
Original Papers
2025
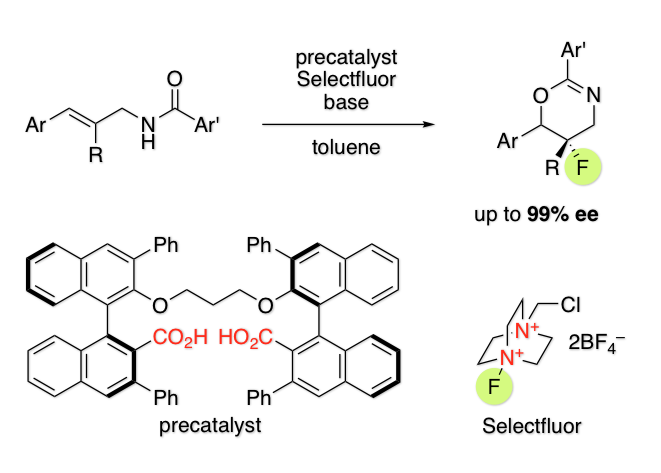
NEW !
Thiobenzoic acid–catalyzed radical–radical cross–coupling between benzyl alcohols and benzophenones via hydrogen atom transfer
R. Osanai, K. Watanabe, H. Egami*, Y. Hamashima*
Tetrahedron Lett. 2025, 169, 155752
DOI:10.1016/j.tetlet.2025.155752

Direct Generation of Carboxyl Radicals from Carboxylic Acids Catalyzed by Photoactivated Ketones
K. Yamashita*, H. Sano, Y. Goto, H. Hayashi*, Y. Hamashima*
J. Am. Chem. Soc. 2025, 147, 33, 29711–29721.
https://doi.org/10.1021/jacs.5c04571
Synfacts
2025,
21(10):,1044
DOI:10.1055/a-2680-9588
.png)
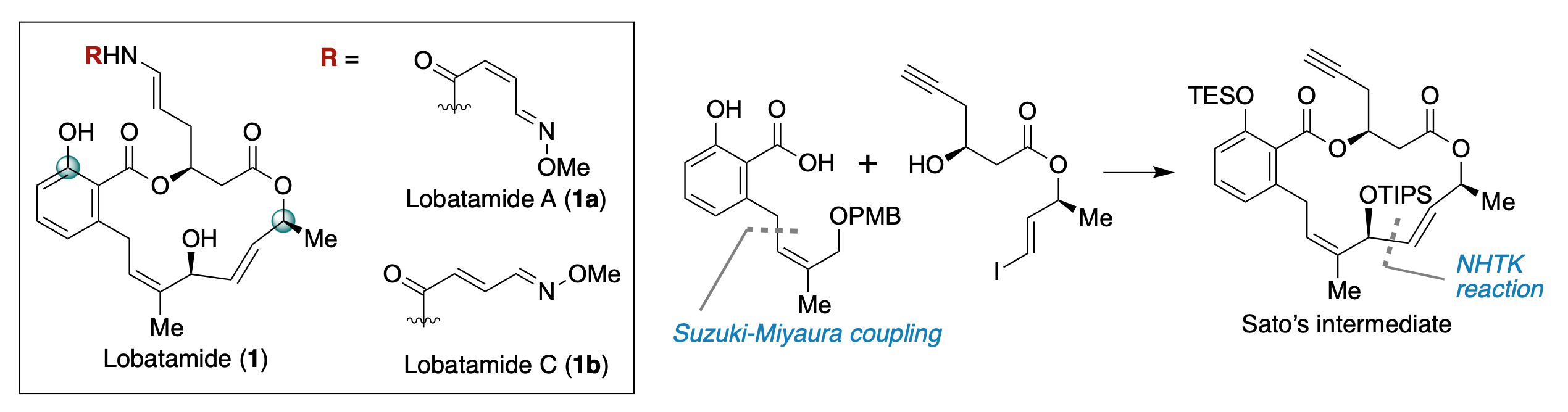
Formal Synthesis of Lobatamides A and C
Y. Nakahara, T. Fukuda, R. Fujii, M. Kanakogi, H. Ouchi, F. Yoshimura, R. Takita, T. Kan, M. Inai*, Y. Hamashima*
Org. Lett. 2025, 27, 12, 3066–3070.
DOI:10.1055/a-2117-8816
2024
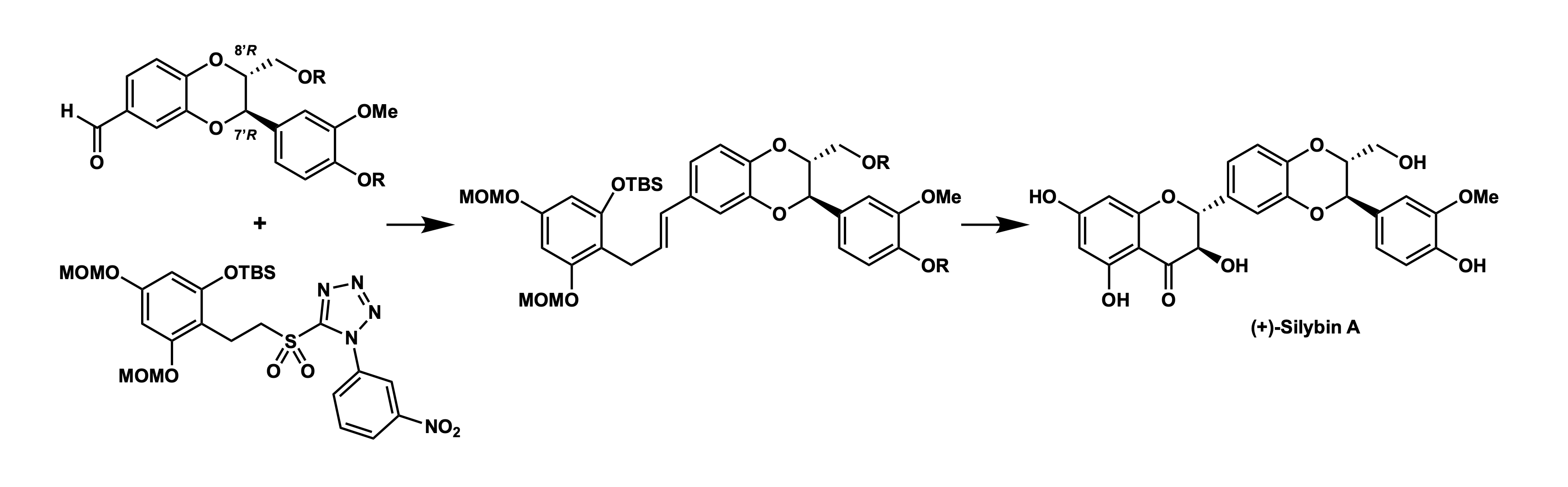
Total Synthesis of (+)-Silybin A
M. Inai*, H. Sagara, Y. Ueno, H. Ouchi, F. Yoshimura, T. Asakawa, Y. Hamashima*, T. Kan
Chem. Pharm. Bull. 2024,
72, 6, 570–573.
DOI:10.1248/cpb.c24-00276
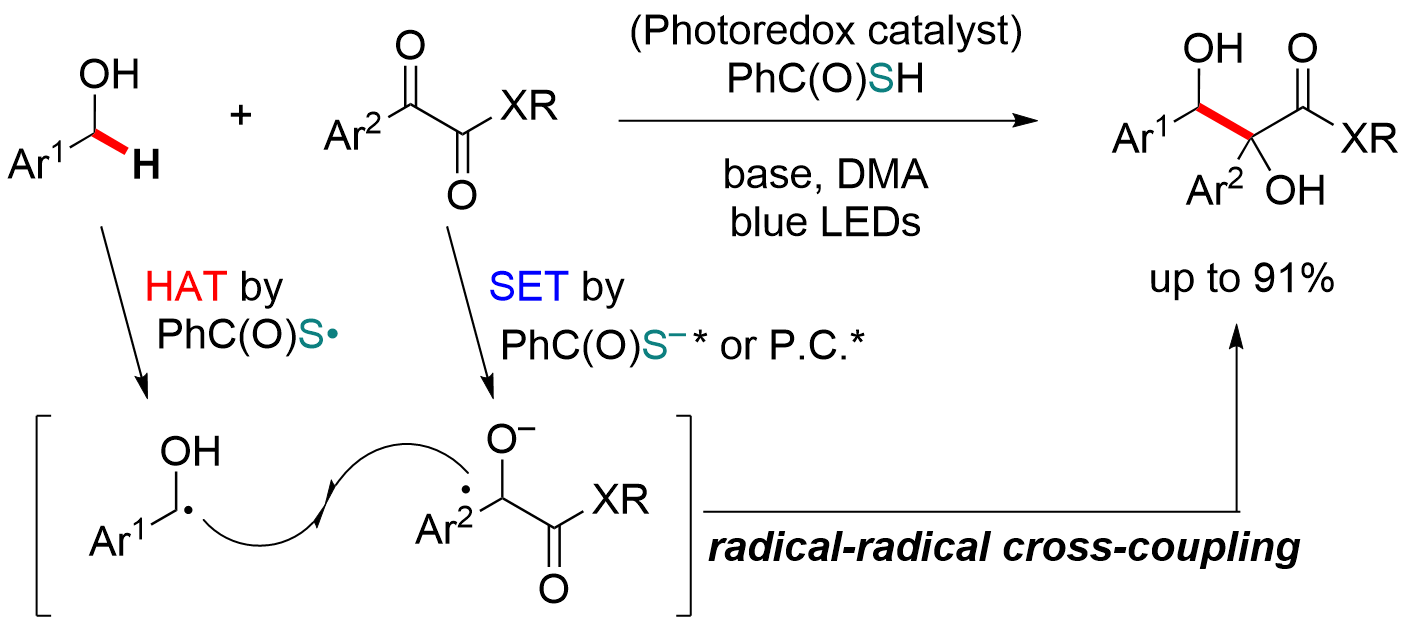
Thiobenzoic Acid-Catalyzed Cα–H Cross Coupling of Benzyl Alcohols with α-Ketoacid Derivatives
K. Sato, H. Egami*, and Y. Hamashima*
Org. Lett. 2024, 26, 25, 5285–5289.
DOI:10.1021/acs.orglett.4c01594
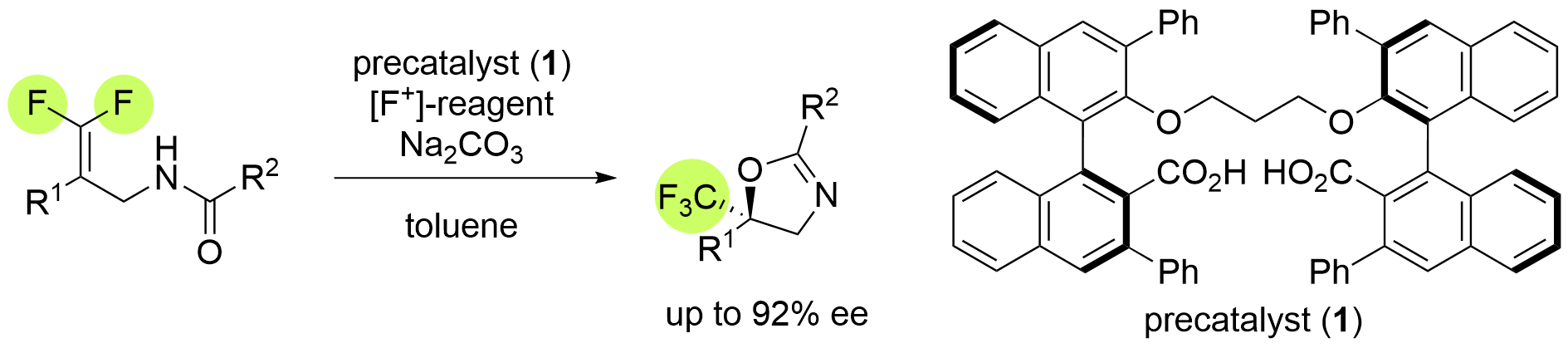
Asymmetric Fluorocyclization of Difluoroalkenes with Concomitant Formation of a Trifluoromethyl Group
C. Igarashi, T. Mayumi, H. Egami*, and Y. Hamashima*
Org. Lett. 2024, 26, 8, 1723–1727.
DOI:10.1021/acs.orglett.4c00312
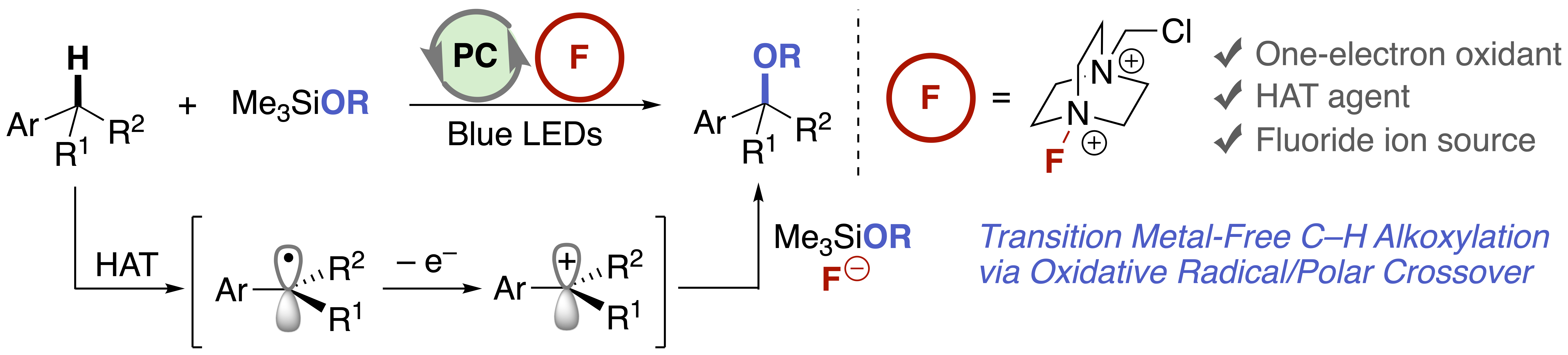
Benzylic C(sp3)–H Alkoxylation through Visible-Light-Driven Oxidative Radical-Polar Crossover
K. Yamashita*, F. Kawahara, and Y. Hamashima*
Asian J. Org. Chem. 2024,
13, 4, e202300662
DOI:10.1002/ajoc.202300662
2023
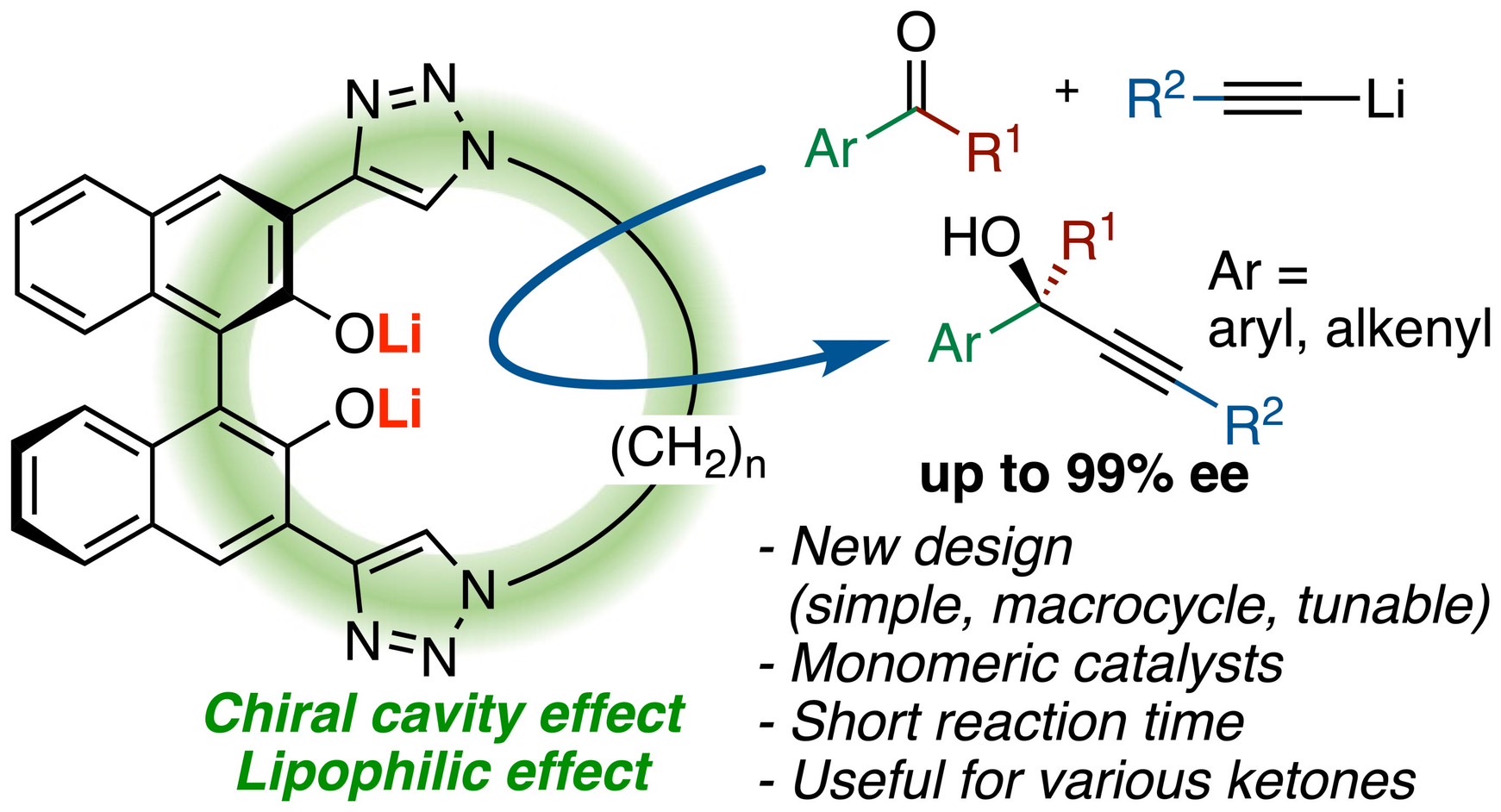
Chiral Macrocyclic Catalysts for the Enantioselective Addition of Lithium Acetylides to Ketones
K. Yamashita, Y. Tabata, K. Yamakawa, T. Mochizuki, K. Matsui, M. Hatano*, and K. Ishihara*
J. Am. Chem. Soc. 2023,
145, 48, 26238–26248.
DOI:10.1021/jacs.3c08905
Charged chiral derivatization for enantioselective imaging of d-,l-2-hydroxyglutaric acid using ion mobility spectrometry/mass spectrometry
E. Sugiyama, Y. Nishiya, K. Yamashita, R. Hirokawa, Y. Iinuma, T. Nirasawa, H. Mizuno, Y. Hamashima, and K. Todoroki*
Chem. Commun.
2023,
59, 10916-10919.
DOI:org/10.1039/d3cc01963b
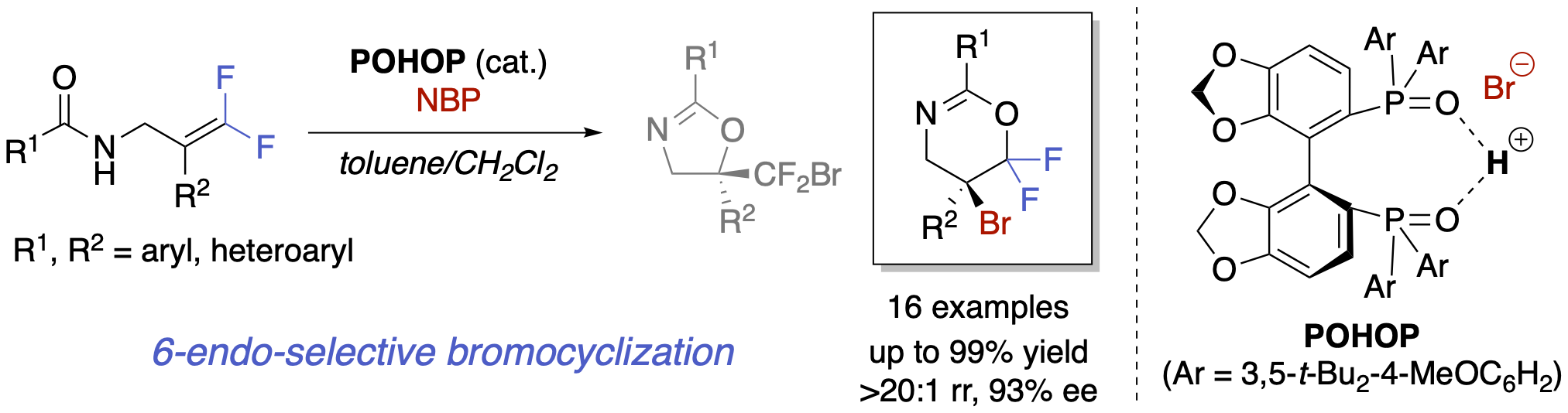
Switching Regioselectivity in the Asymmetric Bromocyclization of Difluoroalkenes Catalyzed by a Chiral Bisphosphine Oxide
Y. Nakahara, R. Hirokawa, S. Uchida, K.Yamashita, and Y. Hamashima*
Synlett
2023,
34, 20, 2476-2480.
DOI:10.1055/a-2117-8816
Experimental and Computational Investigation of Facial Selectivity Switching in Nickel–Diamine–Acetate-Catalyzed Michael Reactions
Y. Sohtome, S. Komagawa, A. Nakamura, D. Hashizume, S. Lectard, M. Akakabe, Y. Hamashima, M. Uchiyama, and M. Sodeoka*
J. Org. Chem.
2023, 88, 7764–7773.
DOI:org/10.1021/acs.joc.2c02732
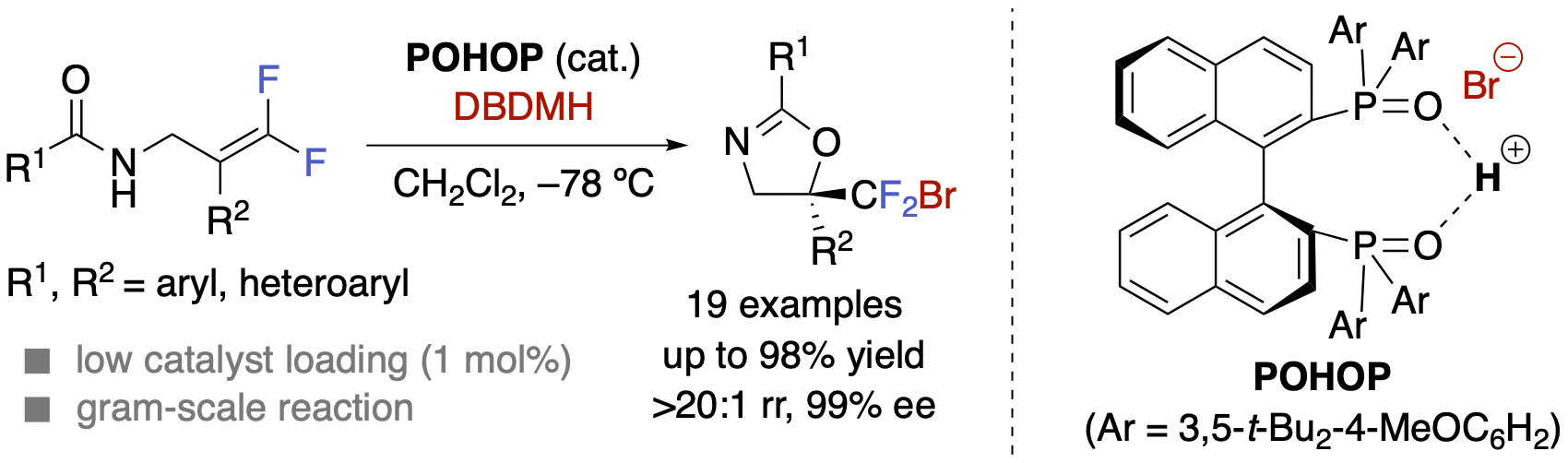
Enantioselective Synthesis of Bromodifluoromethyl-containing Oxazolines by Concerted Lewis/Brønsted Base Catalysis with Chiral Bisphosphine Oxide
R. Hirokawa, Y. Nakahara, S. Uchida, K. Yamashita, and Y. Hamashima
Chem. Asian J. 2023,
18, e202300141.
DOI:org/10.1002/asia.202300141
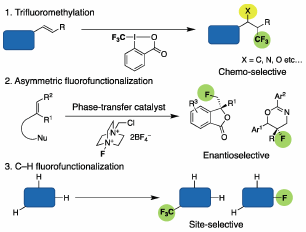
Asymmetric Fluorofunctionalizations with Carboxylate-Based Phase-Transfer Catalysts
H. Egami, and Y. Hamashima*
Chem. Rec.
2023,
23, e202200285.
DOI:org/10.1002/tcr.202200285
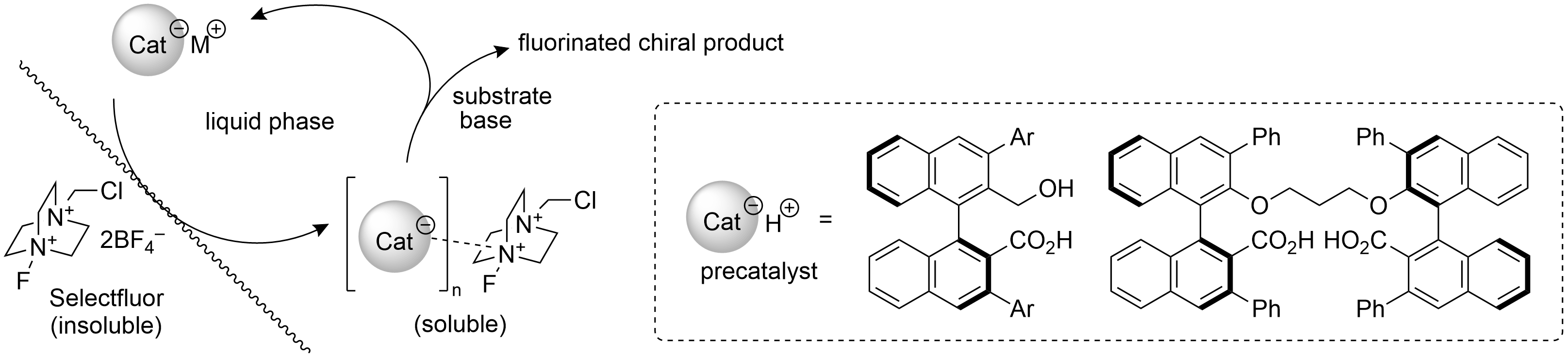
Amide-Ligand-Promoted Silver-Catalyzed C–H Fluorination via Radical/Polar Crossover
K. Yamashita, Y. Fujiwara, and Y. Hamashima*
J. Org. Chem.
2023,
88, 3, 1865–1874.
DOI:org/10.1021/acs.joc.2c02575
2022
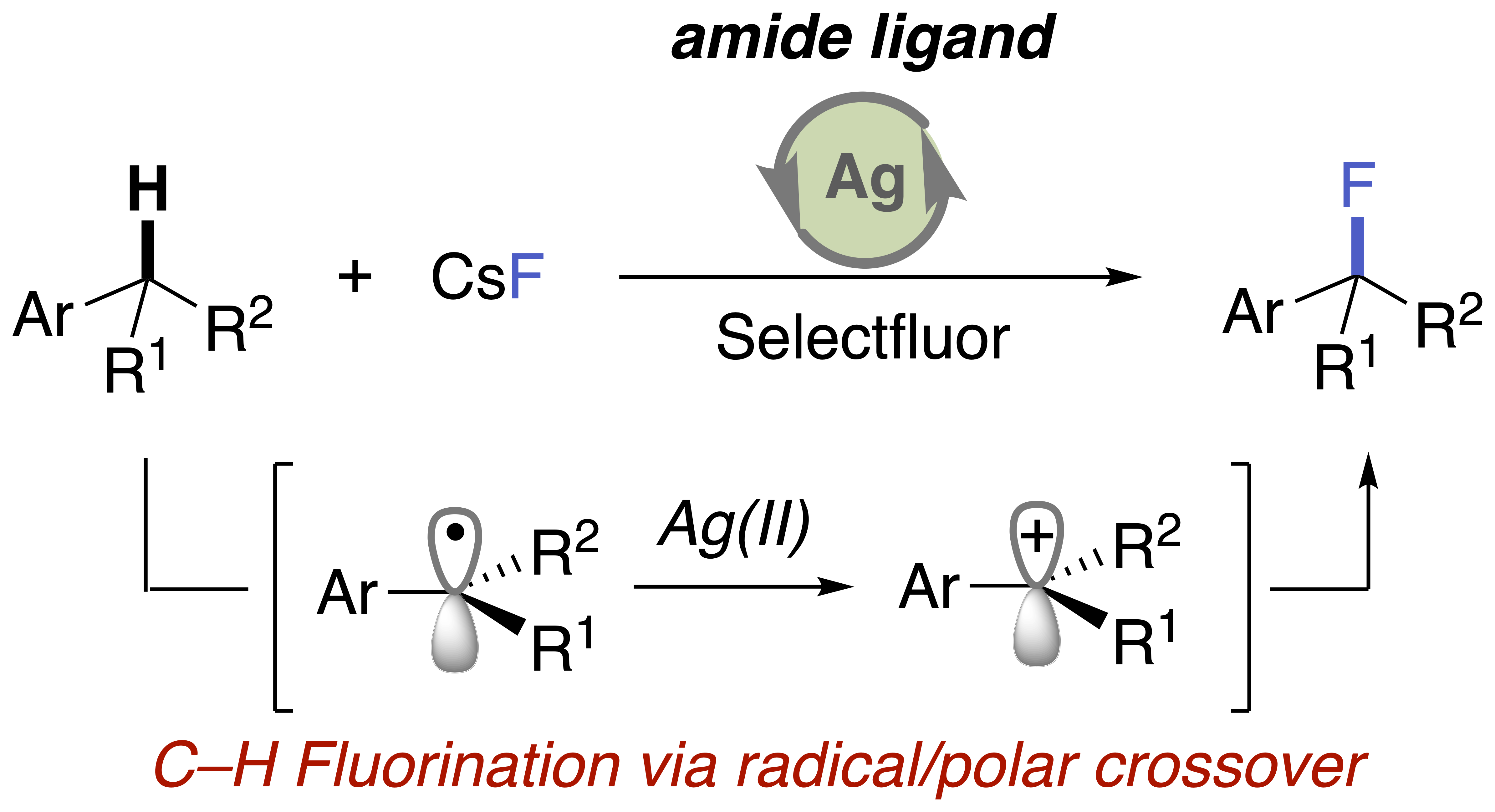
Mechanistic Details of Asymmetric Bromocyclization with BINAP Monoxide: Identification of Chiral Proton-Bridged Bisphosphine Oxide Complex and Its Application to Parallel Kinetic Resolution
K. Yamashita, R. Hirokawa, M. Ichikawa, T. Hisanaga, Y. Nagao, R. Takita, K. Watanabe, Y. Kawato and Y. Hamashima*
J. Am. Chem. Soc.
2022,
144, 9, 3913-3924.
DOI:org/10.1021/jacs.1c11816
2021
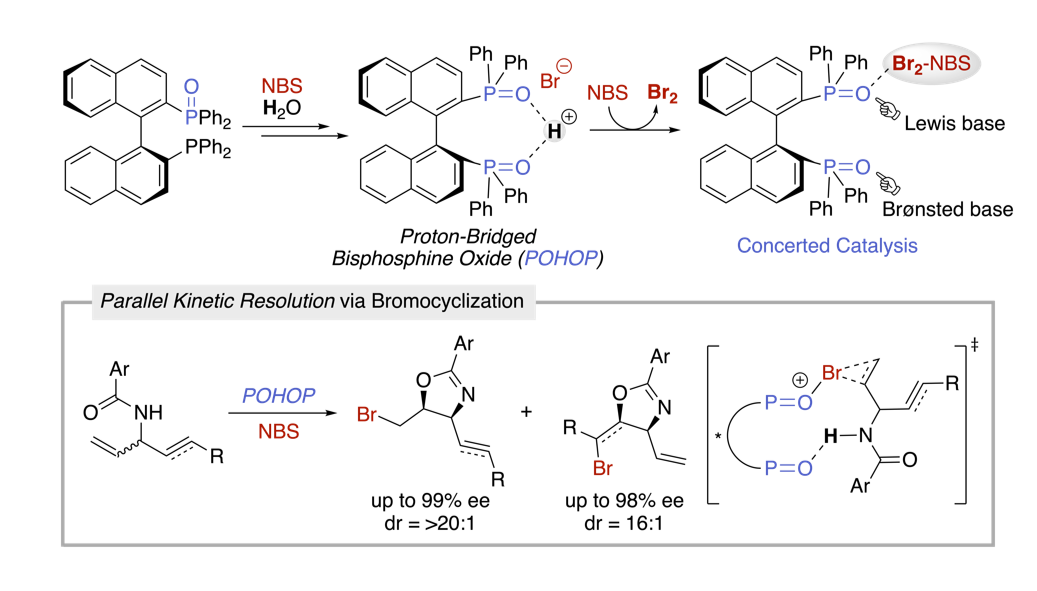
Structure Dependence in Asymmetric Deprotonative Fluorination and Fluorocyclization Reactions of Allylamine Derivatives with Linked Binaphthyl Dicarboxylate Phase-Transfer Catalyst
T. Niwa, K. Nishibashi, H. Sato, K. Ujiie, K. Yamashita, H. Egamiand Y. Hamashima*
J. Am. Chem. Soc.
2021,
143, 40, 16599-16609.
DOI:org/10.1021/jacs.1c06783
Design of synthetic polymer nanoparticles that inhibit glucoseabsorption from the intestine
H. Koide, N. Hayashi, G. Yasuno, A. Okishima, Y. Hoshino, H. Egami, Y. Hamashima, N. Oku, T. Asai*
Biochemical and Biophysical Research Communications
2021, 561, 1-6.
DOI:org/10.1016/j.bbrc.2021.05.005
Dearomative enantio- and diastereoselective difluorination of resorcinol derivatives
M. Otsubo, K. Sakimoto, H. Egami and Y. Hamashima*
Tetrahedron
2021,
96, 132355.
DOI:org/10.1016/j.tet.2021.132355
Enhancement of target toxin neutralization effect in vivo by PEGylation of multifunctionalized lipid nanoparticles
H. Koide, H. Suzuki, H. Ochiai, H. Egami, Y. Hamashima, N. Oku, T. Asai
Biochemical and Biophysical Research Communications
2021,
561, 1-6.
DOI:org/10.1016/j.bbrc.2021.03.073
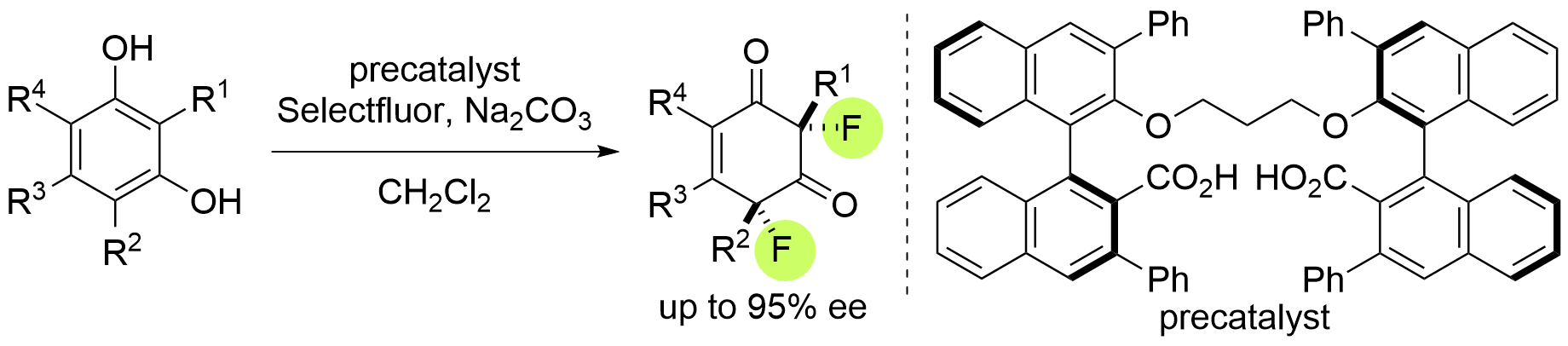
Dual-Role Catalysis by Thiobenzoic Acid in Co-H Arylation under Photoirradiation
F. Kobayashi, M. Fujita, T. Ide, Y. Ito, K. Yamashita, H. Egami, and Y. Hamashima*
Acs Catal.
2021, 11, 82-87.
DOI:10.1021/acscatal.0c04722
2020
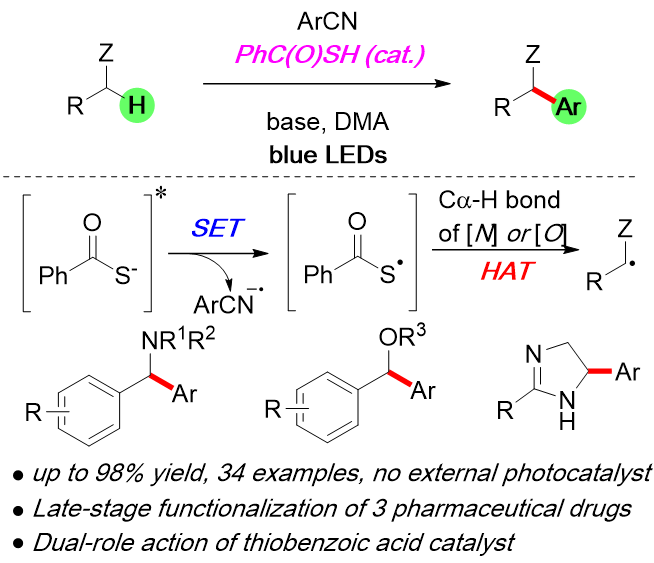
Oxidative and Redox-Neutral Approaches to Symmetrical Diamines and Diols by Single Electron Transfer/Hydrogen Atom Transfer Synergistic Catalysis
M. Fujita, F. Kobayashi, T. Ide, H. Egami, Y. Hamashima*
Eur. J. Org. Chem.
2020, 7151–7155.
DOI:10.1002/ejoc.202001329
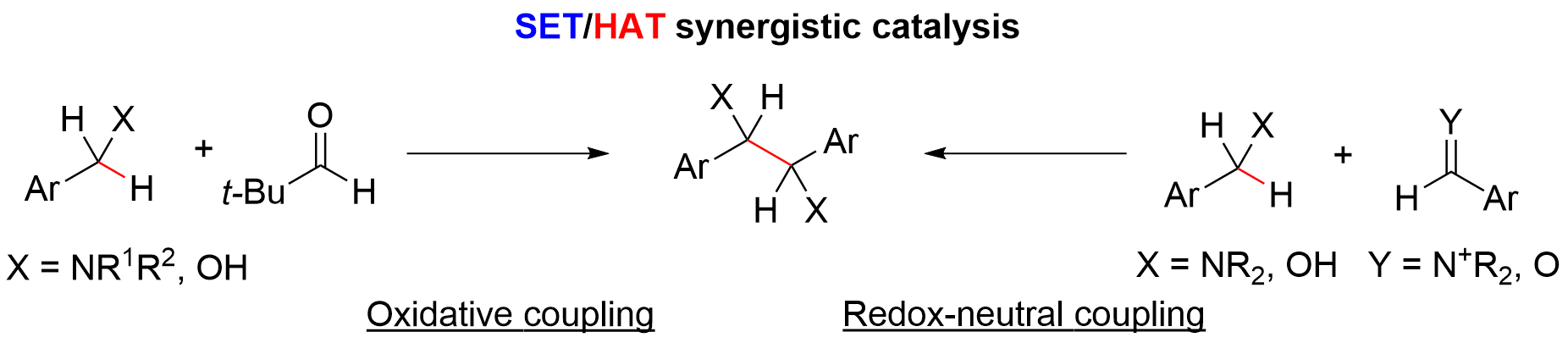
Asymmetric Dearomatizing Fluoroamidation of Indole Derivatives with Dianionic Phase-Transfer Catalyst
H. Egami, R. Hotta, M. Otsubo, T. Rouno, T. Niwa, K. Yamashita and Y. Hamashima*
Org. Lett.
2020,
22, 5656-5660.
DOI:10.1021/acs.orglett.0c02026
Fluorofunctionalizations of C-C Multiple Bonds and C-H Bonds
H. Egami*
Chem. Pharm. Bull.
2020,
68, 491-511.
DOI: 10.1248/cpb.c19-00856
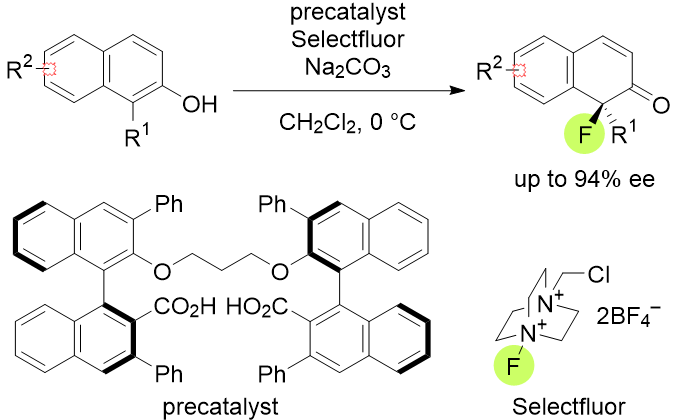
Asymmetric Dearomative Fluorination of 2-Naphthols with Dicarboxylate Phse-Transfer Catalyst
H. Egami, T. Rouno, T. Niwa, K .Masuda, K. Yamashita and Y. Hamashima
Angew. Chem., Int. Ed.
2020,59, 14101-14105.
DOI: 10.1002/anie.202005367
Synfacts
2020,
16(08):,0966
DOI: 10.1055/s-0040-01707436
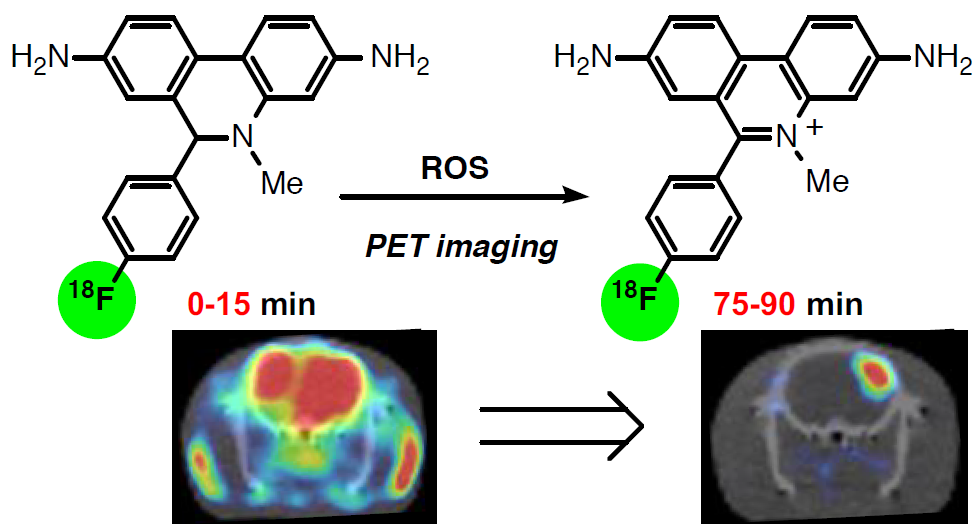
18F-Labeled dihydromethidine: Positron emission tomography radiotracer for imaging of reactive oxygen species in intact brain
H. Egami, S. Nakagawa, Y. Katsura, M. Kanazawa, S. Nishiyama, T. Sakai, Y. Arano, H. Tsukada, O. Inoue, K. Todoroki * and Y. Hamashima*
Org. Biomol. Chem.
2020,
18, 2387-2391.
DOI: 10.1039/D0OB00126K
2019
High Efficiency Microwave Flow Chemistry toward Synthesis of Functional Materials and Pharmaceutical Cores
J. P. Barham, E. Koyama, J. Sugiyama, Y. Norisane, H. Egami and Y. Hamashima*
Ampere
2019, 409-417.
DOI: 10.4995/Ampere2019.2019.9860
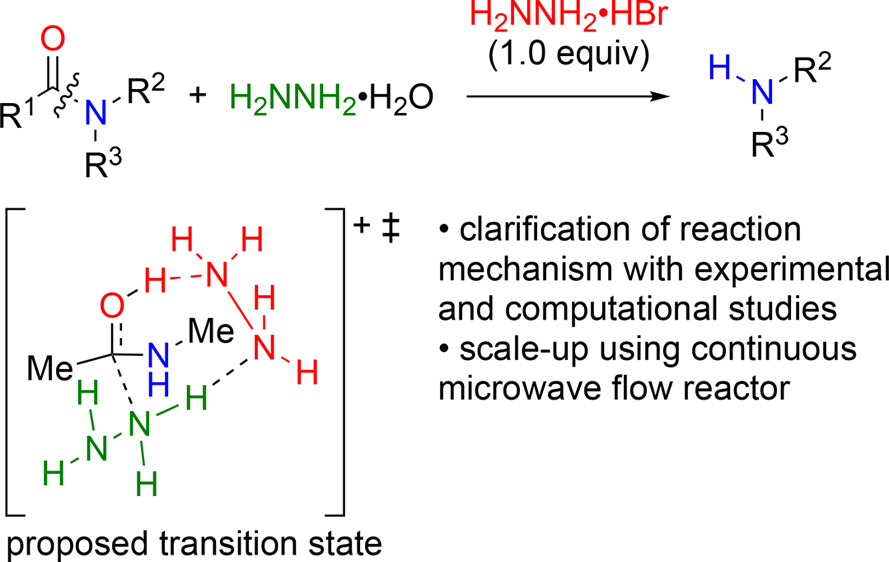
Ammonium Salt-Accelerated Hydrazinolysis of Unactivated Amides: Mechanistic Investigation and Application to a Microwave Flow Process
M. Noshita, Y. Shimizu, H. Morimoto, S. Akai, Y.
Hamashima, N. Ohneda, H. Odajima and T. Ohshima*
Org. Process Res. Dev. 2019, 23, 588-594.
DOI: 10.1021/acs.oprd.8b00424
Consice Synthesis of TAN1251C
Y. Nagasawa, T. Asakawa, S. Shintaku, A. Masuda, K. Matsumura, M. Inai, Y. Ishikawa, M. Egi, Y. Hamashima and T. Kan*
Heterocycles 2019,
99, 1095-1116.
DOI: 10.3987/COM-18-S(F)78
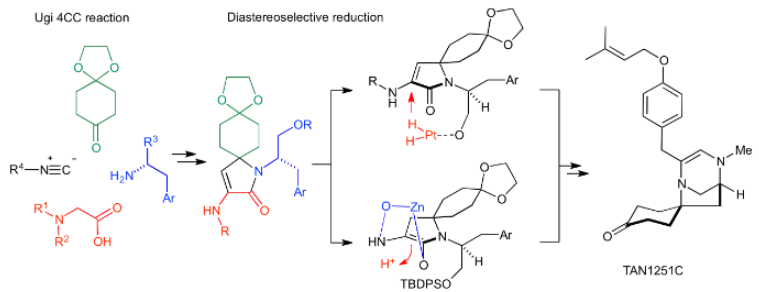
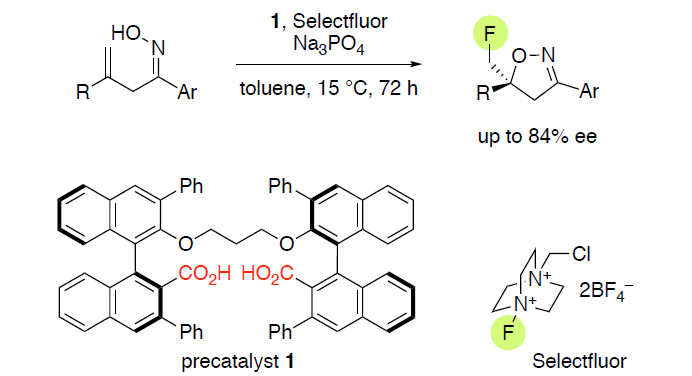
Enantioselective 5-exo-Fluorocyclization of Ene-Oximes
T. Rouno, T. Niwa, K. Nishibashi, N. Yamamoto, H. Egami and Y. Hamashima*
Molecules 2019,
24, 3464.
DOI: 10.3390/molecules24193464
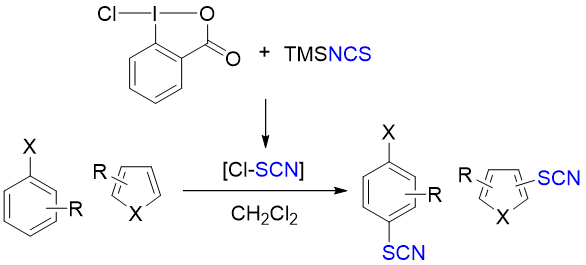
Thiocyanation of Aromatic and Heteroaromatic Compounds with 1-Chloro-1,2-benziodoxol-3-(1H)-one and (Trimethylsilyl)isothiocyanate
Y. Ito, A. Touyama, M. Uku, H. Egami*, and Y. Hamashima*
Chem. Pharm. Bull.
2019,
67, 1015-1018.
DOI:10.1248/cpb.c19-00352
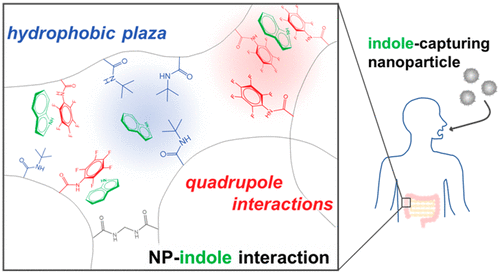
Design of Synthetic Polymer Nanoparticles Specifically Capturing Indole, a Small Toxic Molecule
A. Okishima, H. Koide*, Y. Hoshino, H. Egami, Y. Hamashima, N. Oku, and T. Asai
Biomacromolecules.
2019,
20, 1644–1654.
DOI: 10.1021/acs.biomac.8b01820
Sequestering and inhibiting a vascular endothelial growth factor in vivo by systemic administration of a synthetic polymer nanoparticle
H. Koide, K. Yoshimatsu, Y. Hoshino, S. Ariizumi, A. Okishima, T. Ide, H. Egami, Y. Hamashima, Y. Nishimura, H. Kanazawa, Y. Miura, T. Asai, N. Oku*, and K. J. Shea*
J. Control. Release.
2019,
295, 13-20.
DOI: 10.1016/j.jconrel.2018.12.033

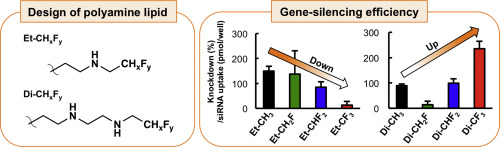
Rigorous control of vesicle-forming lipid pKa by fluorine-conjugated bioisosteres for gene-silencing with siRNA
A. Okamoto, H. Koide, N. Morita, Y. Hirai, Y. Kawato, H. Egami, Y. Hamashima, T. Asai, T. Dewa, and N. Oku*
J. Control. Release.
2019,
295, 87-92.
DOI:10.1016/j.jconrel.2018.12.044
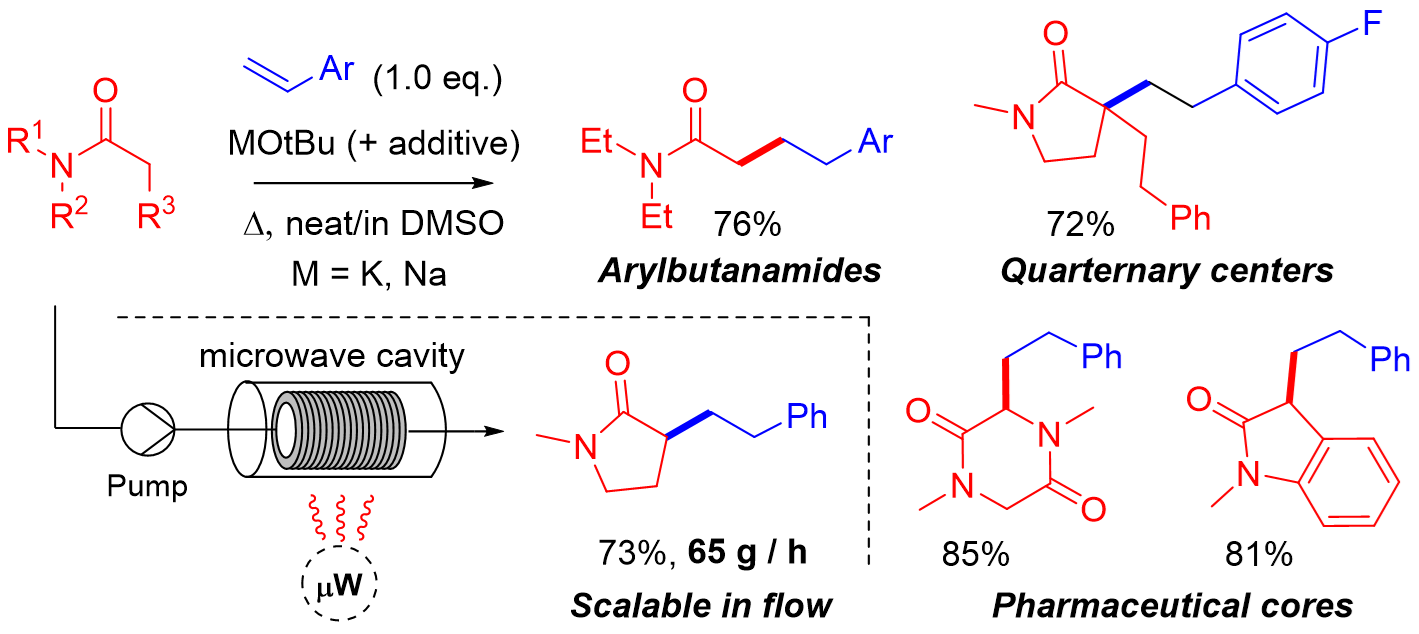
Practical and Scalable Organic Reactions with Flow Microwave Apparatus
H. Egami, and Y. Hamashima*
Chem. Rec.
2019,
19, 157-171.
DOI: 10.1002/tcr.201800132

2018
C-alkylation of N-alkylamides with styrenes in air and scale-up using a microwave flow reactor
J. P. Barham, S, Tamaoki, H. Egami, N, Ohneda, T, Okamoto, H, Odajima, and Y. Hamashima*
Org. Biomol. Chem.
2018,
16,7568-7573.
DOI: 10.1039/C8SC02965B
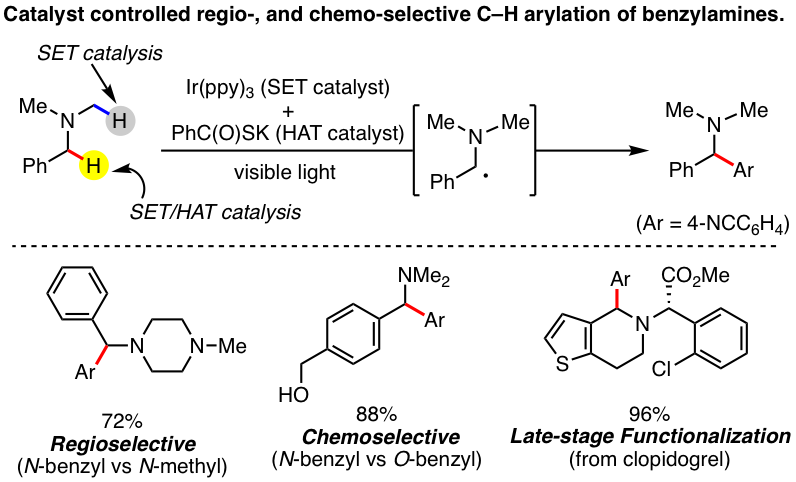
Regio- and chemoselective Csp3–H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis
T. Ide, J. P. Barham, M. Fujita, Y. Kawato, H. Egami, Y. Hamashima*
Chem. Sci.
2018,
9, 8453-8460.
DOI: 10.1039/C8SC02965B
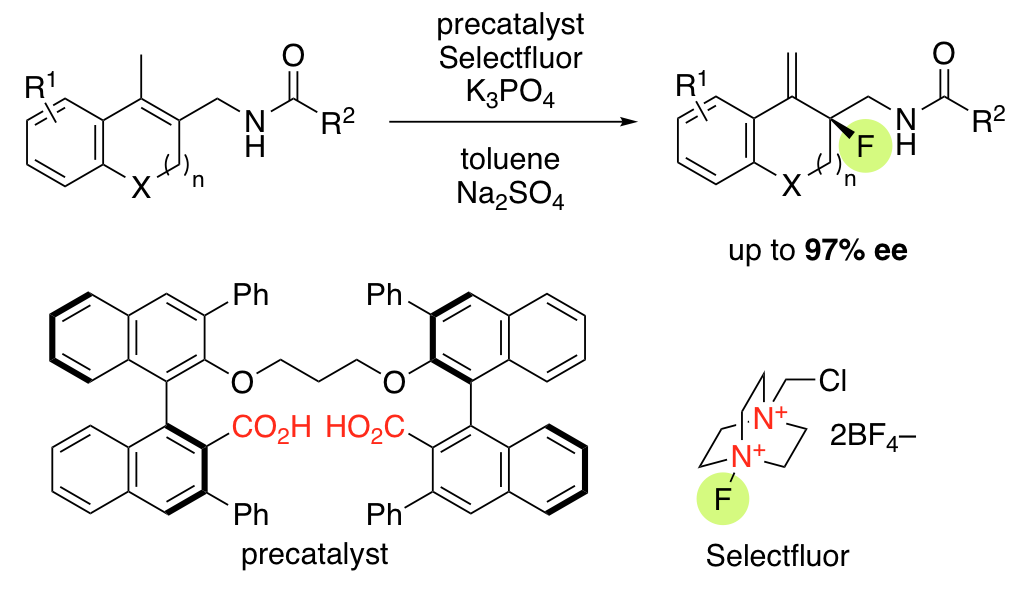
Asymmetric Fluorination of Cyclic Tetrasubstituted Alkenes with a Pendant Amide Groups under Dianionic Phase-transfer Catalysis
T. Niwa, K. Ujiie, H. Sato, H. Egami, Y. Hamashima*
Chem. Pharm. Bull.
2018,
66, 920-922.
DOI:10.1248/cpb.c18-00551
Scalable Microwave-assisted Johnson-Claisen Rearrangement with Continuous Flow Microwave System
H. Egami, S. Tamaoki, M. Abe, N. Ohneda, T. Yoshimura, T. Okamoto, H. Odajima, N. Mase, K. Takeda, Y. Hamashima*
Org. Process Res. Dev.
2018,
22, 1029-1033.
DOI: 10.1021/acs.oprd.8b0018
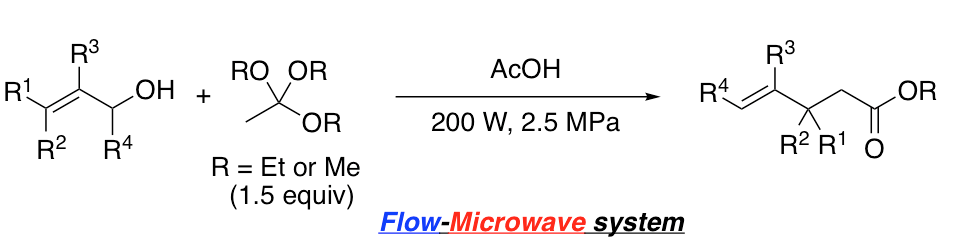
Redox-neutral C−H cyanation of tetrahydroisoquinolines under photoredox catalysis
T. Ide, K. Shimizu, H. Egami, Y. Hamashima*
Tetrahedron Lett.
2018,
59, 3258-3261.
DOI: 10.1016/j.tetlet.2018.07.030
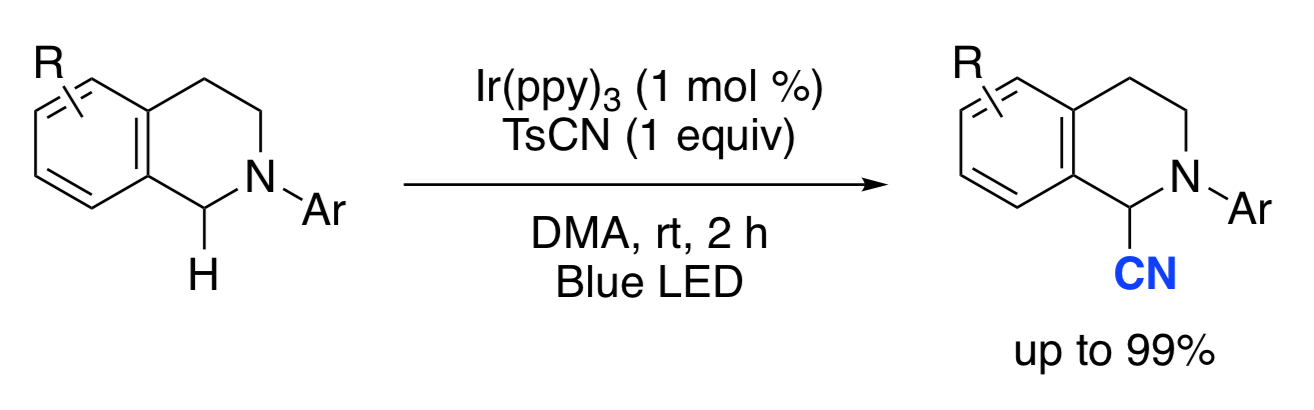
(E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylic Acid
H. Egami, T. Sawairi, S. Tamaoki, N. Ohneda, T. Okamoto, H. Odajima, Y. Hamashima*
Molbank
2018,
M996,
DOI: 10.3390/M996
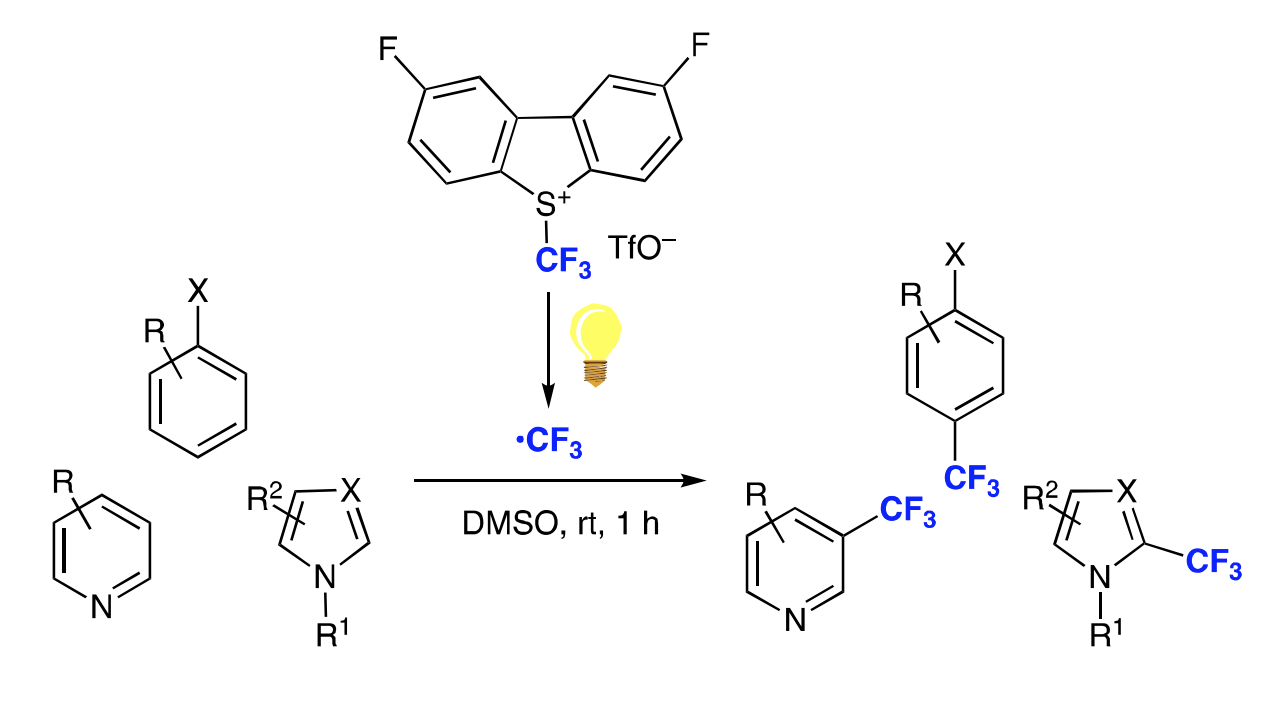
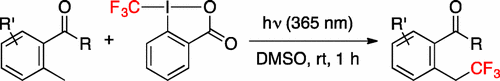
Simple Photo-induced Trifluoromethylation of Aromatic Rings
H. Egami*, Y. Ito, T. Ide, S. Masuda, Y. Hamashima*
Synthesis
2018,
50, 2948-2953.
DOI: 10.1055/s-0037-1609759
Enantioselective Bromocyclization of Allylic Amides Mediated by Phosphorus Catalysis
Y. Kawato, Y. Hamashima*
Synlett
2018,
29, 1257-1271.
DOI: 10.1055/s-0036-1591579
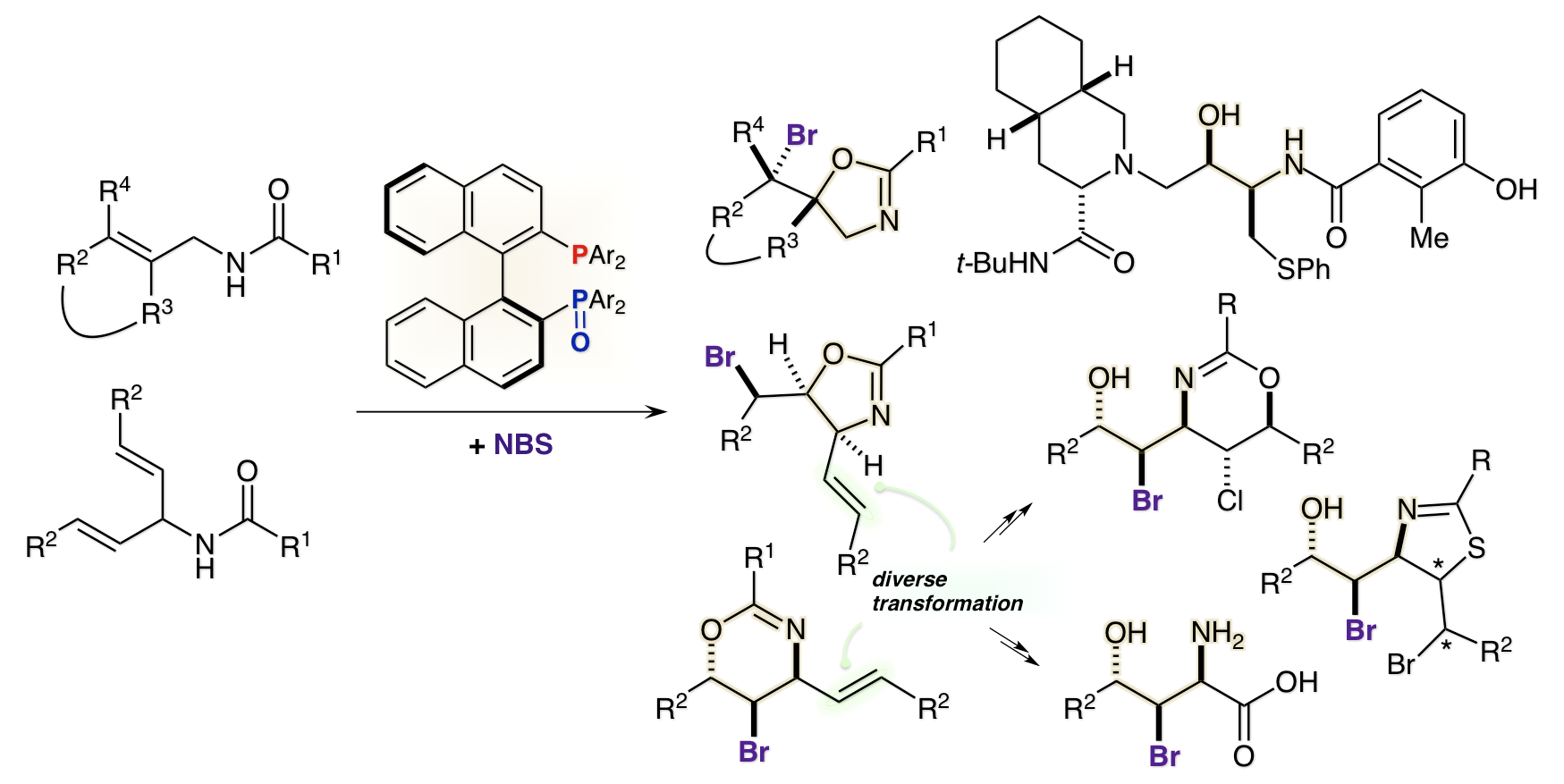
Enantioselective Synthesis of Nelfinavir via Asymmetric Bromocyclization of Bisallylic Amide
Y. Nagao, T. Hisanaga, T. Utsumi, H. Egami, Y. Kawato, Y. Hamashima*
J. Org. Chem.
2018,
83, 7290-7295.
DOI: 10.1021/acs.joc.8b00039
Highlighted in Synfacts
2018, 14, 900
DOI: 10.1055/s-0037-1610560
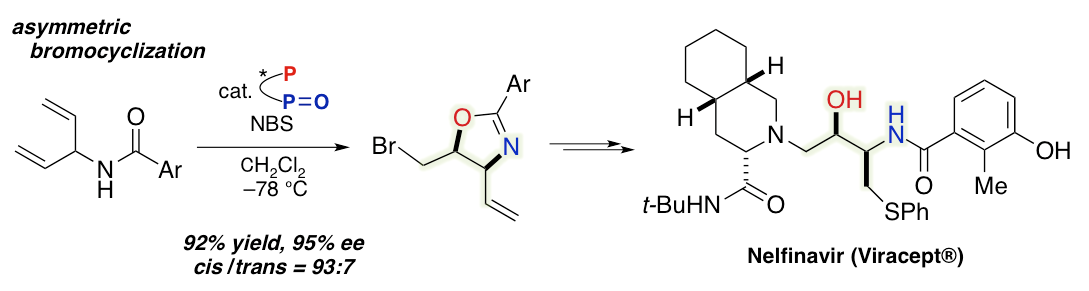
Dianionic Phase-Transfer Catalyst for Asymmetric Fluorocyclization
H. Egami, T. Niwa, H. Sato, R. Hotta, T. Rouno, Y. Kawato, Y. Hamashima*
J. Am. Chem. Soc.
2018,
140, 2785-2788.
DOI: 10.1021/jacs.7b13690
Highlighted in
Synfacts
2018,
14, 534.
DOI: 10.1055/s-0037-1609661
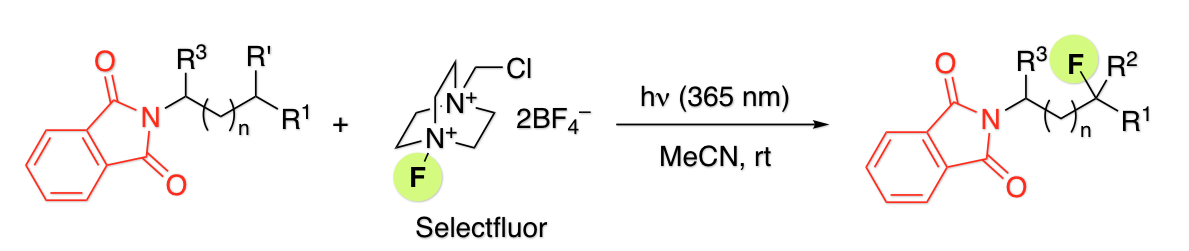
Photofluorination of Aliphatic C-H Bonds Promoted by the Phthalimide Group
H. Egami*, S. Masuda, Y. Kawato, Y. Hamashima*
Org. Lett .
2018,
20, 1367-1370.
DOI: 10.1021/acs.orglett.8b00133
2017
Desymmetrization of Bisallylic Amides through Catalytic Enantioselective Bromocyclization with BINAP Monoxide
Y. Nagao, T. Hisanaga, H. Egami, Y. Kawato, Y. Hamashima*
Chem. Eur. J.
2017,
23, 16758–16762
DOI: 10.1002/chem.201704847 《Most Accessed 11/2017》
Benzylic C-H Trifluoromethylation via Photoenol
T. Ide, S. Masuda, Y. Kawato, H. Egami, Y. Hamashima*
Org. Lett.
2017,
19,4452-4455
DOI: 10.1021/acs.orglett.7b01971
Total Synthesis of TAN1251C via Diastereoselective Construction of the Azaspiro Skeleton
Y. Nagasaka, S. Shintaku, K. Matsumura, A. Masuda, T. Asakawa, M. Inai, M. Egi, Y. Hamashima, Y. Ishikawa, and T. Kan*
Org. Lett.
2017,
19, 3839-3842
DOI: 10.1021/acs.orglett.7b01718
Synthetic Study on Pactamycin: Stereoselective Synthesis of the Cyclopentane Core Framework
A. Goto, S. Yoshimura, Y. Nakao, M. Inai, T. Asakawa, M. Egi, Y. Hamashima, M. Kondo, and T. Kan*
Org. Lett.
2017,
19, 3358-3361
DOI: 10.1021/acs.orglett.7b01257
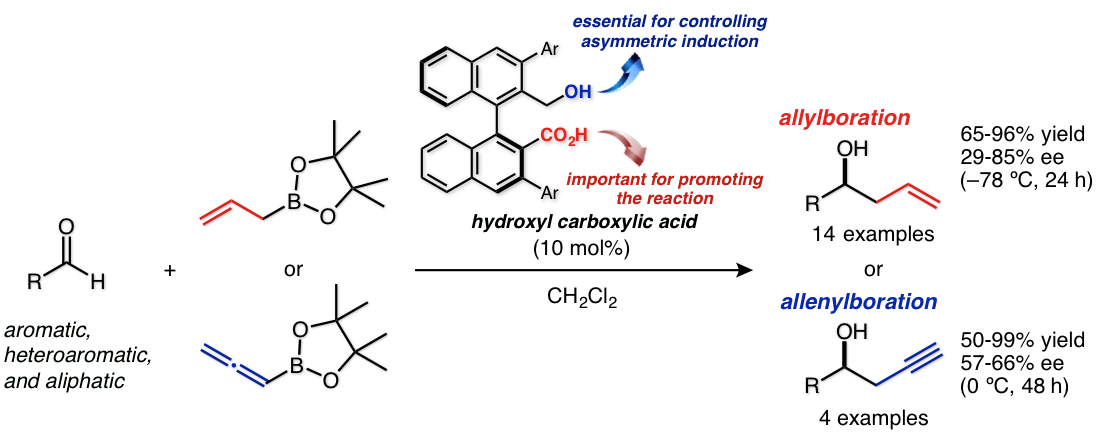
Enantioselective Allyl-, and Allenylboration of Aldehydes Catalyzed by Chiral Hydroxyl Carboxylic Acid
Y. Ota, Y. Kawato, H. Egami, Y. Hamashima*
Synlett
2017,
28, 976-980
DOI: 10.1055/s-0036-1588690
2016
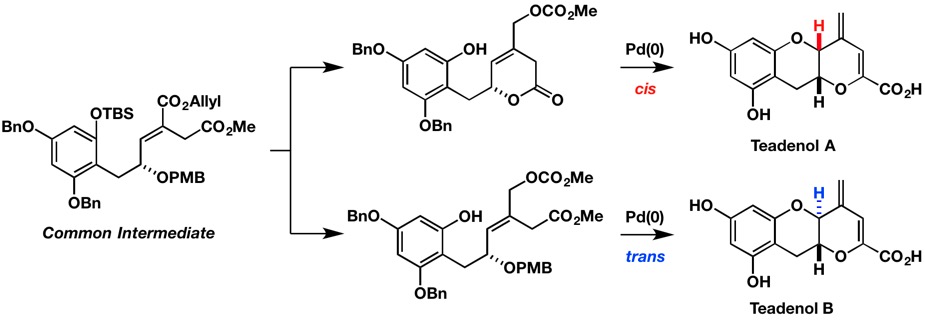
Stereoselective Construction of 2-Vinyl 3-Hydroxybenzopyran Rings: Total Syntheses of Teadenols A and B
R. Yoshida, H. Ouchi, A. Yoshida, T. Asakawa, M. Inai, M. Egi,
Y. Hamashima* and T. Kan*
Org. Biomol. Chem.
2016,
14, 10783-10786
DOI/10.1039/C6OB02004F
α-Functionalization of Tetrahydroisoquinolines with Activated Alkyl Bromide under Photoredox Catalysis
T. Ide, K. Shimizu, Y. Kawato, H. Egami, and Y. Hamashima*
Heterocycles
2017,
95, 738-747.
DOI : 10.3987/COM-16-S(S)73
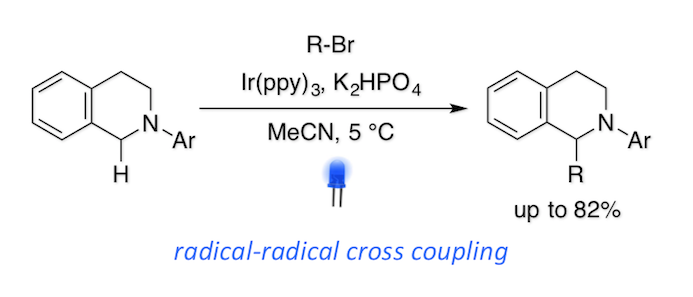
Difunctionalization of Alkenes Using 1-Chloro-1,2-benziodoxol-3-(1H)-one
H. Egami*, T. Yoneda, M. Uku, T. Ide, Y. Kawato, and Y. Hamashima*
J. Org. Chem.
2016
,81, 4020-4030.
DOI/10.1021/acs.joc.6b00295
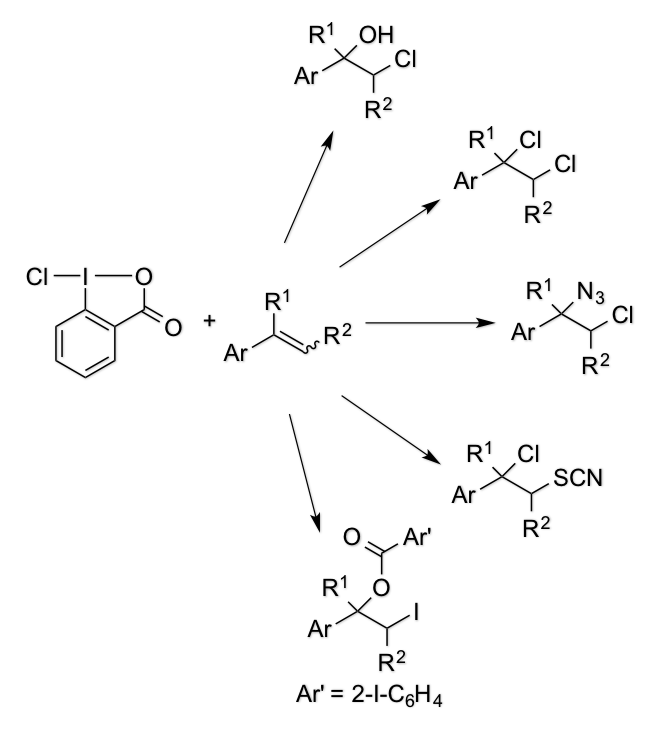
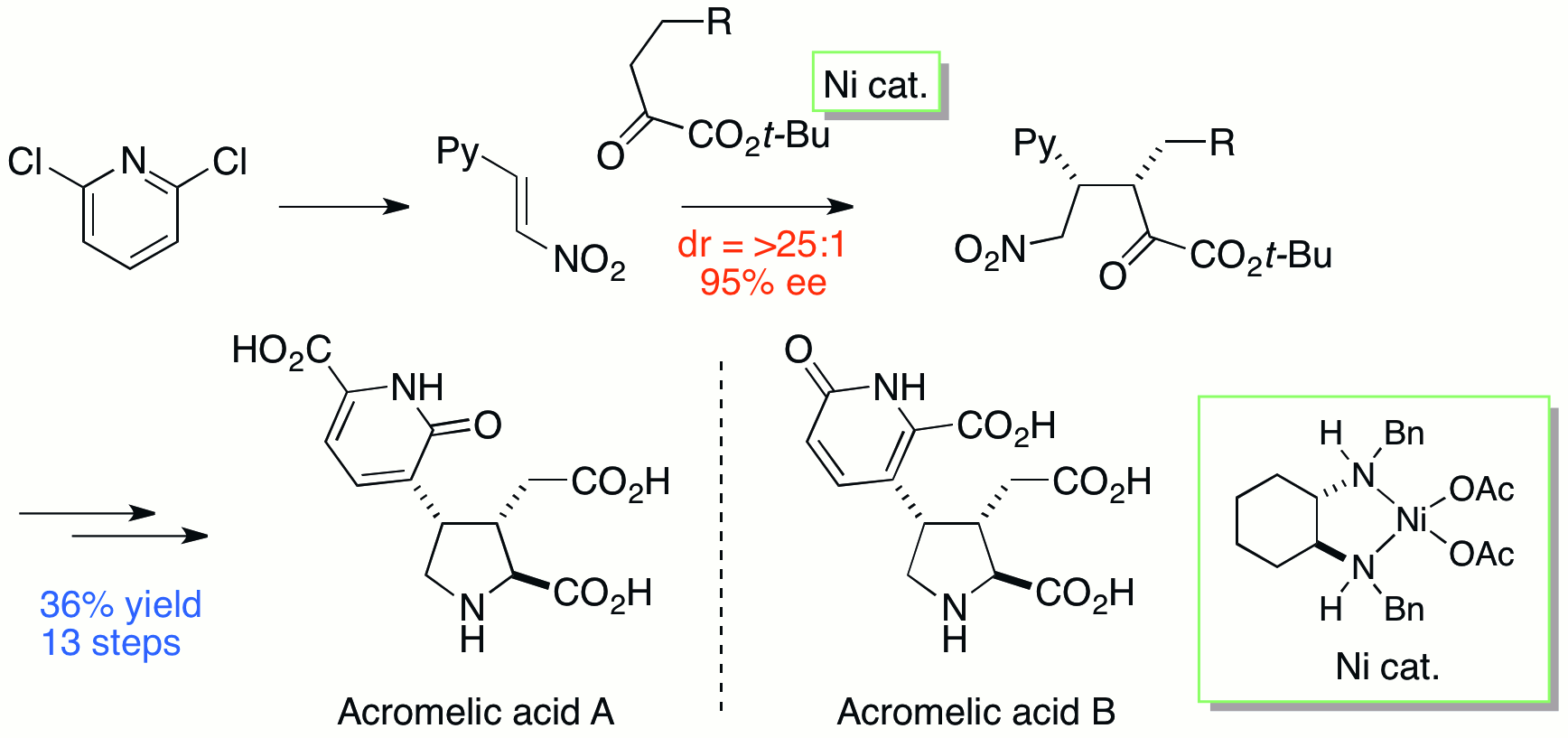
Practical Total Syntheses of Acromelic Acids A and B
M. Inai, H. Ouchi, A. Asahina, T. Asakawa, Y. Hamashima*, and T. Kan*
Chem. Pharm. Bull.
2016,
64, 723-732.
DOI/org/10.1248/cpb.c16-00009
Synthesis of methylated catechins and theaflavins using 2-nitrobenzenesulfonyl group to protect and deactivate phenol
T. Kan, T. Asakawa, Y. Kawabe, A. Yoshida, Y. Aihara, T. Manabe, Y. Hirose, A. Sakurada, M. Inai, Y. Hamashima, T. Furuta, and T. Wakimoto
J.
Antibiot.
2016,
69, 299-312.
DOI/10.1038/ja.2016.14
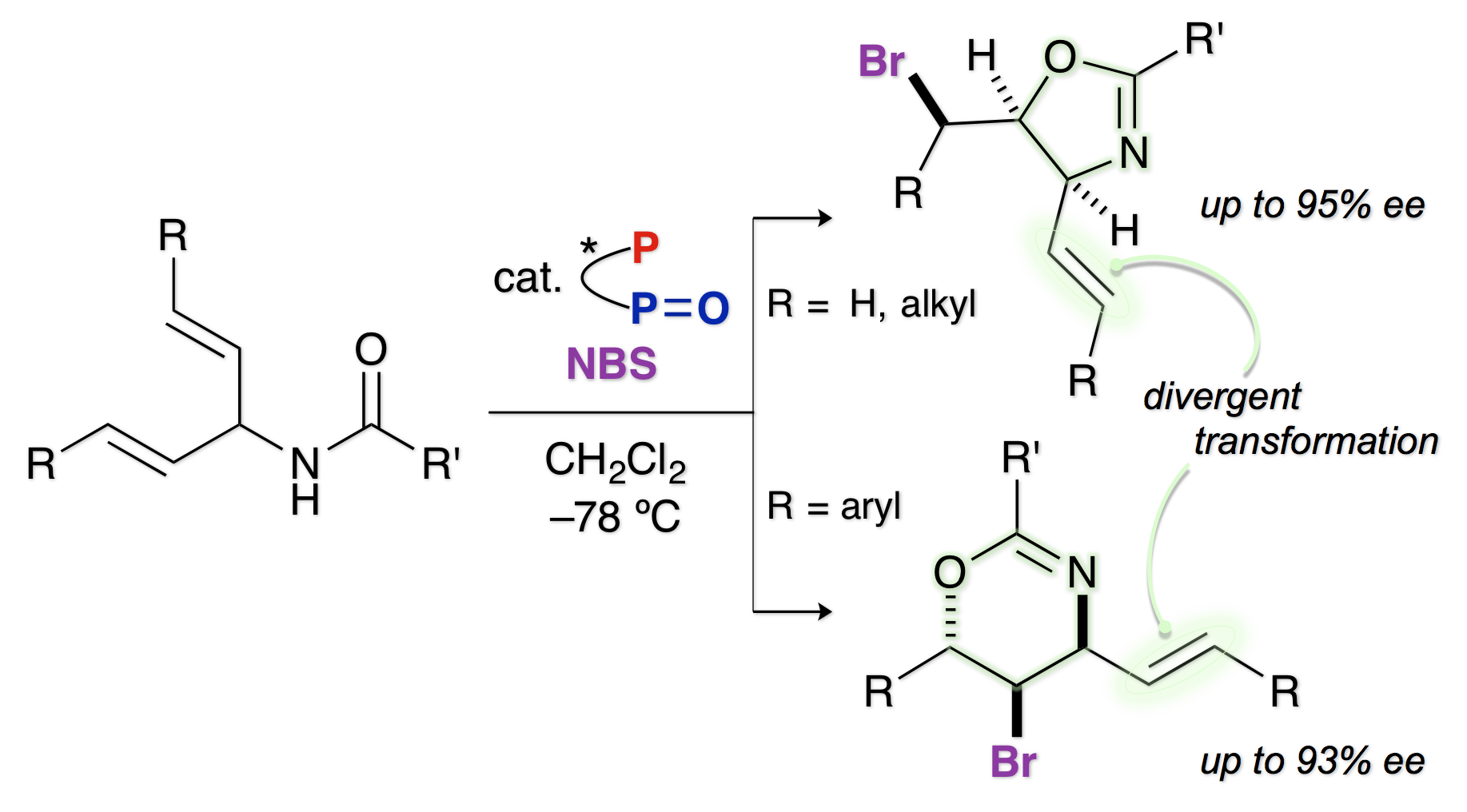
Highly Enantioselective Bromocyclization of Allylic Amides with a P/P=O Double-site Lewis Base Catalyst
Y. Kawato, H. Ono, A. Kubota, Y. Nagao, N. Morita, H. Egami, and Y. Hamashima*
Chem. Eur. J.
2016,
22, 2127-2133.
DOI/10.1002/chem.201503153
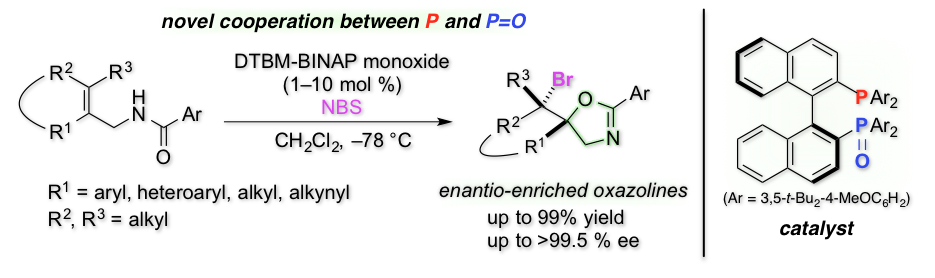
2015
Benzylic C–H Trifluoromethylation of Phenol Derivatives
H. Egami, T. Ide, Y. Kawato, and Y. Hamashima*
Chem. Commun.
2015,
51, 16675–16678.
DOI: 10.1039/C5CC07011B
Asymmetric Fluorolactonization with a Bifunctional Hydroxyl Carboxylate Catalyst
H. Egami, J. Asada, K. Sato, D. Hashizume, Y. Kawato, and Y. Hamashima*
J. Am. Chem. Soc.
2015,
137, 10132–10135.
DOI: 10.1021/jacs.5b06546
Stereocontrolled Total Syntheses of Optically Active Furofuran Lignans
M. Inai, R. Ishikawa, N. Yoshida, N. Shirakawa, Y. Akao, Y. Kawabe, T. Asakawa, M. Egi, Y. Hamashima*, T. Kan*
Synthesis
2015,
47, 3513-3521.
DOI: 10.1055/s-0034-1378812
2-[(Neopentyl glycolato)boryl]phenyl Triflates and Halides for Fluoride-Ion-Mediated Generation of Functionalized Benzynes
T. Ikawa,* R. Yamamoto, A. Takagi, T. Ito, K. Shimizu, M. Goto, Y. Hamashima, S. Akai*
Adv. Synth. Catal.
2015, 357, 2287-2300.
DOI: 10.1002/adsc.201500315
Concise Synthesis of Binaphthol-derived Chiral Dicarboxylic Acids
H. Egami, K. Sato, J. Asada, Y. Kawato, Y. Hamashima*
Tetrahedron 2015, 71, 6384–6388.
DOI:10.1016/j.tet.2015.05.041
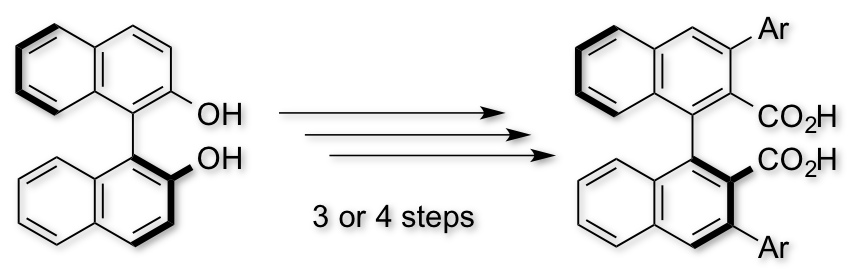
Advanced Dress-up Chiral Columns: New Removable Chiral Stationary Phases for Enantioseparation of Chiral Carboxylic Acids
K. Todoroki, Y. Ishii, T. Ide, J.Z. Min, K. Inoue, X. Huang, W. Zhang, Y. Hamashima, T. Toyo'oka*
Analytica Chimica Acta 2015, 882, 101-111.
DOI:org/10.1016/j.aca.2015.03.037
Enantioselective Bromocyclization of Allylic Amides Catalyzed byBINAP Derivatives
Y. Kawato, A. Kubota, H. Ono, H. Egami, Y, Hamashima*
Org. Lett. 2015, 17, 1244-1247.
DOI:10.1021/acs.orglett.5b00220
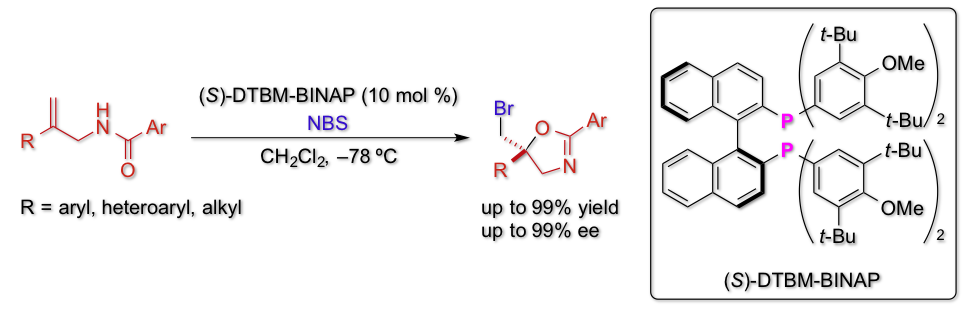
Development of a Highly Efficient Single-mode Microwave Applicator with a Resonant Cavity and Its Application to Continuous Flow Syntheses
S. Yokosawa, N. Ohneda, K. Muramatsu, T. Okamoto, H, Odashima, T. Ikawa, J. Sugiyama, M. Fujita, T. Sawairi, H. Egami, Y, Hamashima, M. Egi, S. Akai*
RSC Adv. 2015, 5, 10204-10210.
DOI:10.1039/C4RA12428F
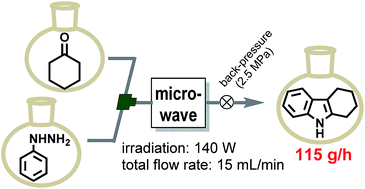
2014
Dual Catalysis with Copper and Rhenium for Trifluoromethylation of Propargylic Alcohols: Efficient Synthesis of α-Trifluoromethylated Enones
H. Egami, T. Ide, M. Fujita, T. Tojo, Y, Hamashima*, M.
Sodeoka*
Chem. Eur. J. 2014, 20, 12061–12065.
DOI:10.1002/chem.201403447
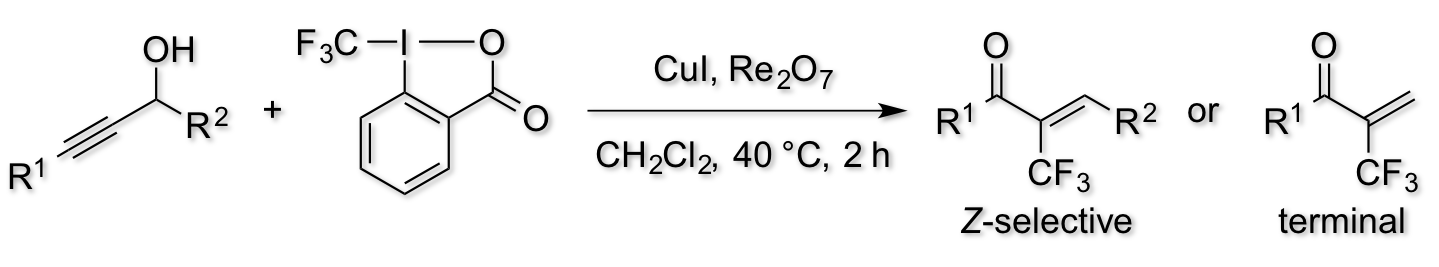
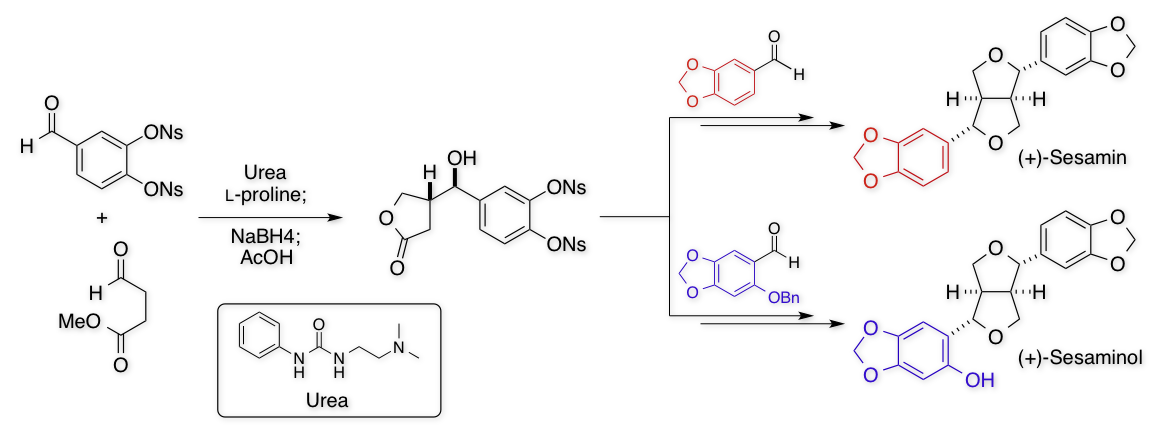
Total Syntheses of (+)-Sesamin and
(+)-Sesaminol
R. Ishikawa, N. Yoshida, Y.Akao, Y. Kawabe, M. Inai, T. Asakawa, Y.
Hamashima*, T. Kan*
Chem. Lett. 2014, 43, 1572-1574.
DOI:10.1246/cl.140613
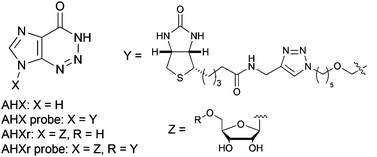
Practical synthesis of natural
plant-growth regulator
2-azahypoxanthine, its derivatives, and biotin-labeled probes
K. Ikeuchi, R. Fujii, S. Sugiyama, T. Asakawa, M. Inai, Y. Hamashima,
J. H. Choi, T. Suzuki, H. Kawagishi*, T. Kan*
Org. Biomol. Chem., 2014, 12, 3813-3815.
DOI:10.1039/C4OB00705K
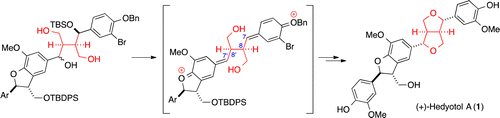
Stereocontrolled Total Synthesis of
Hedyotol A
Y. Kawabe, R. Ishikawa, Y. Akao, A. Yoshida, M. Inai, T. Asakawa, Y.
Hamashima*, T. Kan*
Org. Lett., 2014, 16, 1976-1979.
DOI:10.1021/ol500524y
Practical Total Syntheses of
Acromelic Acids A and B
H. Ouchi, A. Asahina, T. Asakawa, M. Inai, Y. Hamashima*, T.
Kan*
Org. Lett., 2014, 16, 1980-1983.
DOI:10.1021/ol500529w
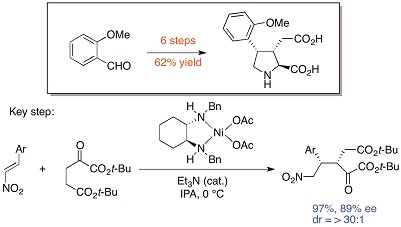
Practical Synthesis of Kainoids: A New Chemical Probe Precursor and a Fluorescent Probe
S. Sasaki, H. Suzuki, H. Ouchi, T. Asakawa, M. Inai, R. Sakai, K.
Shimamoto, Y. Hamashima*, T. Kan*
Org. Lett. 2014, 16, 564-567.
DOI:10.1021/ol403434e
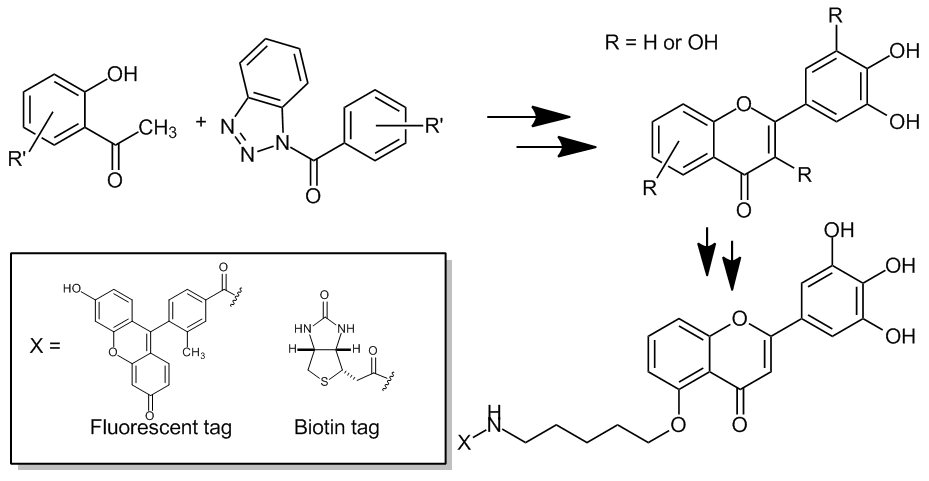
Synthetic Studies of Fisetin, Myricetin and Nobiletin Analogs and Related Probe Molecules
A. Hiza, Y. Tsukaguchi, T. Ogawa, M. Inai, T. Asakawa, Y. Hamashima*,
T.
Kan*
HETEROCYCLES 2014, 88, 1371-1396.
DOI:10.3987/COM-13-S(S)107
2013
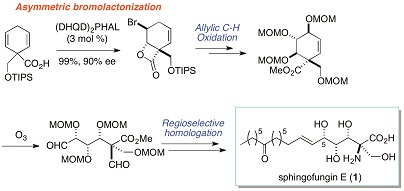
Stereocontrolled total synthesis of sphingofungin E
K. Ikeuchi, M. Hayashi, T. Yamamoto, M. Inai, T. Asakawa, Y. Hamashima, T. Kan
Eur. J. Org. Chem. 2013, 30, 6789-6792.
DOI:10.1002/ejoc.201301065
Chemoselective Hydrogenation Reaction of Unsaturated Bonds in the Presence of o-Nitrobenzenesulfonyl Group
A. Kawanishi, C. Miyamoto, Y. Yabe, M. Inai, T. Asakawa, Y. Hamashima, H. Sajiki, T. Kan*
Org. Lett. 2013, 15, 1306-1309.
DOI:10.1021/ol4002448
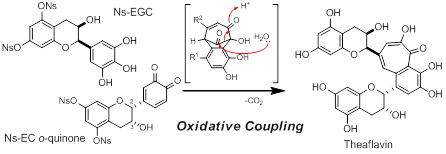
Synthesis of Theaflavin via Biomimetic Oxidative Coupling Reaction
Y. Kawabe, Y. Aihara, Y. Hirose, A. Sakurada, A. Yoshida, M. Inai, T. Asakawa, Y. Hamashima*, T. Kan*
Synlett 2013, 479-482.
DOI:10.1055/s-0032-1318131
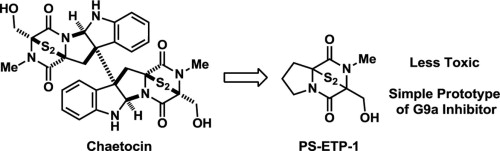
Epidithiodiketopiperazine as a pharmacophore for protein lysine methyltransferase G9a inhibitors: Reducing cytotoxicity by structural simplification
S. Fujishiro, K. Dodo, E. Iwasa, Y. Sohtome, Y. Hamashima, A. Ito, M. Yoshida, M. Sodeoka*
Bioorg. Med. Chem. Lett. 2013, 23, 733-736.
DOI:10.1016/j.bmcl.2012.11.087
2012
Catalytic Desymmetrization of Cyclohexadienes via Asymmetric Bromolactonization
K. Ikeuchi, K. Ido, S. Yoshimura, T. Asakawa, M. Inai, Y. Hamashima*, T. Kan*
Org. Lett. 2012, 14, 6016-6019.
Stereocontrolled Total Synthesis of (+)- UCS1025A
K. Uchida, T. Ogawa, Y. Yasuda, H. Mimura, T. Fujimoto, T. Fukuyama, T.
Wakimoto, T. Asakawa, Y. Hamashima*, T. Kan*
Angew. Chem., Int. Ed. 2012, 51, 12850-12853.
Total Synthesis of (-)-Lemonomycin
A. Yoshida, M. Akaiwa, T. Asakawa, Y. Hamashima, S. Yokoshima, T.
Fukuyama, T. Kan*
Chem. Eur. J. 2012, 18, 11192-11195.
A Short-step Asymmetric Synthesis of Dehydrodiconifery Alcohol (DCA)
via C-H Insertion Reaction
S. Matsumoto, T. Asakawa, Y. Hamashima*, T. Kan*
Synlett 2012, 1082-1084.
Copper-Catalyzed Trifluoromethylation of Allylsilanes
R. Shimizu, H. Egami, Y. Hamashima*, M. Sodeoka*
Angew. Chem., Int. Ed. 2012, 51, 4577-4580.
Catalytic Asymmetric Mono-fluorination of a-Keto Ester: Synthesis of
Optically
Active a-Hydroxy-b-fluoro Esters and a-Amino-b-fluoro Esters
S. Suzuki, Y. Kitamura, S. Lectard, Y. Hamashima*, M. Sodeoka*
Angew. Chem., Int. Ed. 2012, 51, 4581-4585.
Synthesis and Biological Activities of Chaetocin and its Derivatives
M. Sodeoka, K. Dodo, Y. Teng, K. Iuchi, Y. Hamashima, E. Iwasa, S.
Fujishiro
Pure Appl. Chem. 2012, 84, 1369-1378.
For before 2011__PDF。
Reviews and Accounts
Chemical synthesis of tea
polyphenols and related compounds
T. Asakawa, Y. Hamashima, T. Kan
Current Pharmaceutical Design 2013, 19,
6207-6217.
Epipolythiodiketopiperazine
Alkaloids: Total Syntheses and
Biological Activities
E. Iwasa, Y. Hamashima, M. Sodeoka
Israel J. Chem. 2011, 51, 420-433.
Recent Advances in Catalytic
Enantioselective Fluorination Reactions
S. Lectard, Y. Hamashima, M. Sodeoka
Adv. Synth. & Catal. 2010, 352, 2708-2732.
Development of Novel Catalytic
Asymmetric Reactions Using Cationic
Group-10 Metal Complexes: With a Special Focus on Reactions in which
Palladium Enolates Plays a Key Role
Y. Hamashima, M. Sodeoka
TCI MAIL 2010, 140, 2-16.
Chiral Pd aqua complex-catalyzed
asymmetric C-C bond-forming
reactions: a Brønsted acid-base cooperative system
M. Sodeoka, Y. Hamashima
Chem. Commun. 2009, 5787-5798.
Catalytic Enantioselective
a-Fluorination of Carbonyl Compounds
Using Chiral Transition Metal Complexes
Y. Hamashima, M. Sodeoka
J. Synth. Org. Chem., Jpn. 2007, 65, 1099-1107.
Acid-Base Catalysis of Chiral
Palladium Complexs: Development of
Novel Asymmetric Reactions and Their Application to Synthesis of Drug
Candidates
Y. Hamashima
Chem. Pharm. Bull. 2006, 54, 1351-1364.
Enantioselective Fluorination
Reactions Catalyzed by Chiral
Palladium Complexes
Y. Hamashima, M. Sodeoka
Synlett 2006, 1467-1478.
Development of Catalytic Asymmetric
Reactions via Chiral Palladium
Enolates
Y. Hamashima, M. Sodeoka
Chem. Rec. 2004, 4, 231-242.
Book Chapters
Carbon-Carbon Bond-Forming
Reactions via Transmetallation Using
Silyl Enol Ethers
Y. Hamashima, M. Sodeoka
In Comprehensive Chirality (E. M. Carreira, H. Yamamoto et al. Eds.:
Elsevier), Vol. 4, 210-213 (2012).
Michael Addition Reactions
Y. Hamashima, M. Sodeoka
In Handbook of C-H Transformations (G. Dyker, Ed.; Wiley-VHC),
Vol. 2, 347-359 (2005)

