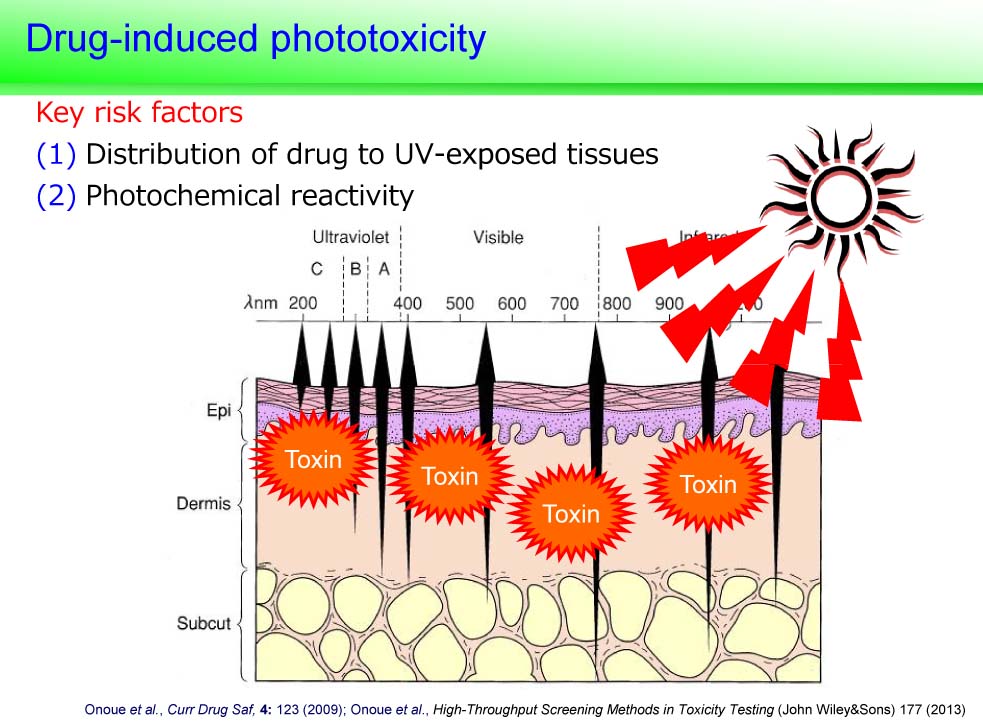
Research projects
1. DDS development to avoid systemic side-effects
Example “Inhalable Powder Formulation of Pirfenidone with Reduced Phototoxic Risk for Treatment of Pulmonary Fibrosis”
Orally-taken pirfenidone (PFD), an idiopathic pulmonary fibrosis drug, often causes severe phototoxicity. Previously, we developed a respirable powder formulation for PFD (PFD-RP) to minimize phototoxic risk. Laser diffraction and cascade impactor analyses on PFD-RP suggested its high dispersion and fine in vitro inhalation performance. Inhaled PFD-RP could suppress antigen-evoked pulmonary inflammation in rats as evidenced by decreases in recruited inflammatory cells and neutrophilia-related biomarkers in the lung. Exposure of PFD to light-exposed tissues (skin and eye) after intratracheal administration of PFD-RP at a pharmacologically effective dose was 90–130-fold less than that of the oral PFD dosage form at a phototoxic dose. Herein, the PFD-RP might be an attractive alternative to the current oral PFD therapy with a better safety margin.
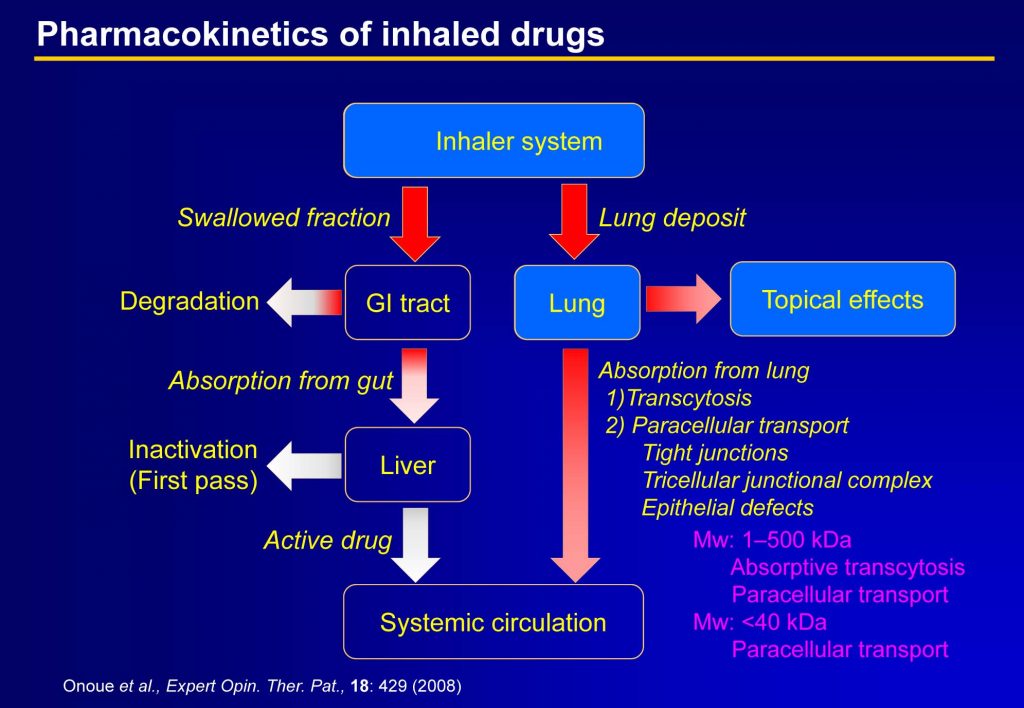
2. Nanodrugs to improve efficacy and pharmacokinetic behavior of pharmaceutical candidates
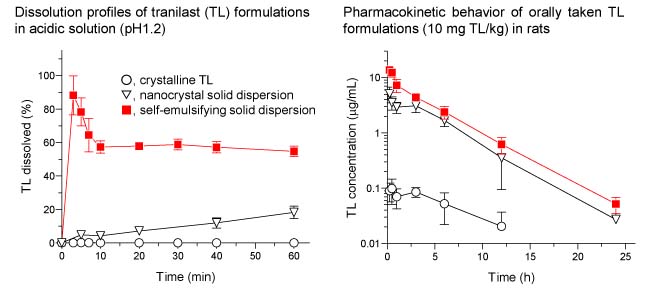
Example “Nano-crystalline Solid Dispersion of Tranilast with High Photostability and Improved Oral Bioavailability”
Tranilast (TL) is an anti-allergic agent and widely used in the clinical treatment of bronchial asthma, atopic rhinitis, atopic dermatitis and keloids. However, therapeutic potential of TL could be partly limited because of its poor solubility, bioavailability, and photostability. To overcome these drawbacks, nano-crystalline solid dispersion of TL (CSD/TL) was prepared by wet-milling technique with aim of improving physicochemical and pharmacokinetic properties. TL particles in CSD/TL appeared to be crystalline with diameter of 122 nm, and CSD/TL exhibited marked improvement in the dissolution behavior as compared to crystalline TL. After oral administration of CSD/TL, enhanced TL exposure was observed with increase of Cmax and AUC by 60- and 32-fold, respectively, as compared to crystalline TL. Therefore, nano-crystal solid dispersion strategy would be efficacious to enhance bioavailability of TL with high photochemical stability.

3. Characterization of pharmacokinetic alteration of drugs under specific pathological condition and its avoidance by DDS strategy
Example “Poor Oral Bioavailability of Dipyridamole under Hypochlorhydria and its improvement by Microenvironmental pH-modifier Approach”
Dipyridamole (DP) is a pH-dependent poorly soluble drug, and previous studies in healthy elderly patients also demonstrated that increasing gastric pH with H2 receptor antagonist dramatically decreased DP absorption. We employed an acidic pH-modifier for improving dissolution and absorption of DP under hypochlorhydric condition. The granule formulation of DP (DPG) was prepared by employing fumaric acid (FA) as a microenvironmental pH-modifier, allowing a pH-independent dissolution. Even in the presence of fumaric acid, no significant chemical or photochemical degradation of DP was seen in the DPG with FA under accelerated condition or UV irradiation. There appeared to be poor and inconsistent systemic exposure of DP early after oral administration of DPG in the hypochlorhydric rats; however, improved pharmacokinetic behavior of DP with low inter-individual variation was observed after oral administration of the DPG with FA. The pH-independent dissolution of DPG with FA might explain in part the high absorption under gastric hypochlorhydria. From these findings, the use of pH-modifier might be efficacious for increasing the therapeutic potential of DP in the hypochlorhydric patients.


4. Pharmacokinetic and formulation studies on nutraceuticals for improved activity
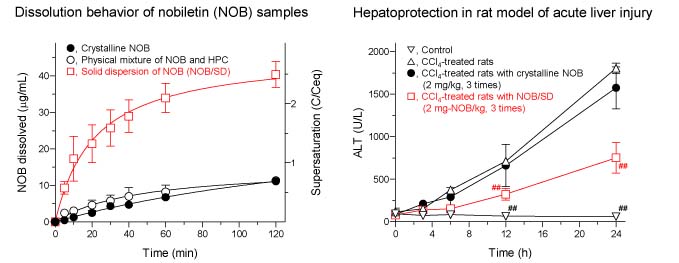
Example “Amorphous Solid Dispersion of Nobiletin, a Citrus Polymethoxylated Flavone, with Improved Biopharmaceutical Properties”
Previously, we developed an amorphous solid dispersion (SD) of nobiletin (NOB), a citrus polymethoxylated flavone, with the aim of improving its biopharmaceutical and hepatoprotective properties. During the storage of NOB/SD for 4 weeks under accelerated conditions, there were no significant transitions in the appearance, particle size, and amorphousity of wet-milled NOB. In comparison with crystalline NOB, the NOB/SD exhibited significant improvement in the dissolution with a 10-fold higher dissolution rate. In a rat model of acute liver injury, repeated treatment with NOB/SD (2 mg NOB/kg) every 4 h led to marked attenuation of hepatic damage as evidenced by decreased ALT and AST, surrogate biomarkers for hepatic injury; however, crystalline NOB was found to be less effective. After oral administration of NOB/SD (2 mg NOB/kg) in rats, compared with crystalline NOB, improved pharmacokinetic behavior was observed with increases of bioavailability and hepatic delivery by ca. 7- and 6-fold, respectively, possibly leading to better hepatoprotection. Given the improved physicochemical and biopharmaceutical properties, the SD formulation strategy might be efficacious for enhancing the therapeutic potential of NOB.

5. Strategic prediction of side-effect on the basis of physicochemical and pharmacokinetic properties
Example “Photosafety Screening of Pharmaceutical Substances with Combined Use of Photochemical and Cassette-dosing Pharmacokinetic Data”
We developed reactive oxygen species (ROS) assay for photosafety assessment that was designed to monitor generation of ROS from the tested chemicals under exposure to simulated sunlight. The phototoxic chemicals tended to generate ROS when exposed to simulated sunlight, and the standard assay protocol was already validated under collaboration with NIHS/JaCVAM and JPMA. Also, we established an efficient photosafety screening system, employing in vitro photochemical and cassette-dosing pharmacokinetic (PK) studies. The established strategy was partly accepted in ICH S10 guideline.